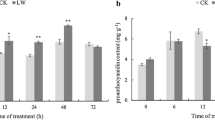
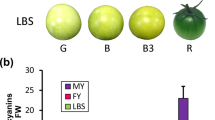
Abstract
In sweet cherry, as in most non-climacteric species, abscisic acid (ABA) plays a major role in the control of fruit ripening and color development. Although the ABA treatment of sweet cherry fruits has been reported to upregulate anthocyanin pathway-related genes or ABA pathway-related genes, the temporality of molecular and physiological events occurring during color development and the ABA control of these events during the color initiation are lacking in this species. In this work, we analyzed variations in the Index of Absorbance Difference (IAD), a maturity index, and total anthocyanins along with changes in transcript abundance of ABA and anthocyanin pathway-related genes, from light green to red fruit stages. PavNCED1 and ABA signaling pathway-related genes upregulated when fruits transitioned from light green to pink stage, whereas anthocyanin pathway-related transcripts increased from pink to the red stage, together with increases in the anthocyanin content and IAD, suggesting sequentiality in molecular and physiological events during color development. Additionally, ABA applied at color initiation in planta advanced IAD, increased anthocyanin content, and yielded darker fruits at harvest. These changes were accompanied by changes in the transcript accumulation of ABA and anthocyanin pathway-related genes. This in planta treatment of sweet cherry fruits with ABA confirms that ABA is a central player in the control of color initiation in sweet cherries, associated with the transcript accumulation of genes involved in ABA homeostasis and signaling, which is followed by the up-regulation of anthocyanin pathway-related genes and color development.



Similar content being viewed by others
References
Alkio M, Jonas U, Declercq M, Van Nocker S, Knoche M (2014) Transcriptional dynamics of the developing sweet cherry (Prunus avium L.) fruit: sequencing, annotation and expression profiling of exocarp-associated genes. Hortic Res 1(1):11. https://doi.org/10.1038/hortres.2014.11
Arapitsas P, Perenzoni D, Nicolini G, Mattivi F (2012) Study of Sangiovese wines pigment profile by UHPLC-MS/MS. J Agric Food Chem 60(42):10461–10471. https://doi.org/10.1021/jf302617e
Betemps DL, Fachinello JC, Galarca SP, Portela NM, Remorini D, Massai R, Agati G (2012) Non-destructive evaluation of ripening and quality traits in apples using a multiparametric fluorescence sensor. J Sci Food Agric 92(9):1855–1864. https://doi.org/10.1002/jsfa.5552
Böttcher C, Keyzers RA, Boss PK, Davies C (2010) Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J Exp Bot 61(13):3615–3625. https://doi.org/10.1093/jxb/erq174
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55(4):611–622. https://doi.org/10.1373/clinchem.2008.112797
Chavoshi M, Watkins C, Oraguzie B, Zhao Y, Iezzoni A, Oraguzie N (2014) Phenotyping protocol for sweet cherry (Prunus avium L.) to facilitate an understanding of trait inheritance. J Am Pom Soc (APS) 68(3):125–134. https://www.pubhort.org/aps/68/v68_n3_a2.htm
Cherian S, Figueroa CR, Nair H (2014) ‘Movers and shakers’ in the regulation of fruit ripening: a cross-dissection of climacteric versus non-climacteric fruit. J Exp Bot 65(17):4705–4722. https://doi.org/10.1093/jxb/eru280
Cheung YK, Klotz JH (1997) The Mann Whitney Wilcoxon distribution using linked lists. Statistica Sinica 7(3):805–813
Coombe BG (1976) The development of fleshy fruits. Annu Rev Plant Physiol 27(1):207–228. https://doi.org/10.1146/annurev.pp.27.060176.001231
Costa G, Vidoni S, Rocchi L (2016) Use of non-destructive devices to support pre- and postharvest fruit management. Acta Hortic 1119:329–335. https://doi.org/10.17660/ActaHortic.2016.1119.45
Fadón E, Herrero M, Rodrigo J (2015) Flower development in sweet cherry framed in the BBCH scale. Sci Hortic 192:141–147. https://doi.org/10.1016/j.scienta.2015.05.027
Gambetta GA, Matthews MA, Shaghasi TH, McElrone AJ, Castellarin SD (2010) Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta 232(1):219–234. https://doi.org/10.1007/s00425-010-1165-2
Giovannoni J (2001) Molecular biology of fruit maturation and ripening. Annu Rev. Plant Physiol 52:725–749. https://doi.org/10.1146/annurev.arplant.52.1.725
Guo X, Wang Y, Zhai Z, Huang T, Zhao D, Peng X, Feng C, Xiao Y, Li T (2018) Transcriptomic analysis of light-dependent anthocyanin accumulation in biocolored cherry fruits. Plant Physiol Biochem 130:663–677. https://doi.org/10.1016/j.plaphy.2018.08.016
Hardner CM, Hayes BJ, Kumar S, Vanderzande S, Cai L, Piaskowski J, Quero-Garcia J, Campoy JA, Barreneche T, Giovannini D, Liverani A, Charlot G, Villamil-Castro M, Oraguzie N, Peace CP (2019) Prediction of genetic value for sweet cherry fruit maturity among environments using a 6K SNP array. Hortic Res 6:6. https://doi.org/10.1038/s41438-018-0081-7
Jia HF, Chai YM, Li CL, Lu D, Luo JJ, Qin L, Shen YY (2011) Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol 157(1):188–199. https://doi.org/10.1104/pp.111.177311
Jin W, Wang H, Li M, Wang J, Yang Y, Zhang X, Yan G, Zhang H, Liu J, Zhang K (2016) The R2R3 MYB transcription factor PavMYB10.1 involves in anthocyanin biosynthesis and determines fruit skin colour in sweet cherry (Prunus avium L.). Plant Biotechnol J 14(11):2120–2133. https://doi.org/10.1111/pbi.12568
Kondo S, Gemma H (1993) Relationship between abscisic acid (ABA) content and maturation of the sweet cherry. J Jpn Soc Hortic Sci 62(1):63–68. https://doi.org/10.2503/jjshs.62.63
Kondo S, Inoue K (1997) Abscisic acid (ABA) and 1-aminocyclopropane-l-carboxylic acid (ACC) content during growth of ‘Satohnishiki’ cherry fruit, and the effect of ABA and ethephon application on fruit quality. J Hortic Sci Biotechnol 72(2):221–227. https://doi.org/10.1080/14620316.1997.11515509
Kuhn N, Guan L, Dai ZW, Wu BH, Lauvergeat V, Gomes E, Li SH, Godoy F, Arce-Johnson P, Delrot S (2014) Berry ripening: recently heard through the grapevine. J Exp Bot 65(16):4543–4559. https://doi.org/10.1093/jxb/ert395
Kuhn N, Ponce C, Arellano M, Time A, Sagredo B, Donoso JM, Meisel LA (2020) Gibberellic acid modifies the transcript abundance of ABA pathway orthologs and modulates sweet cherry (Prunus avium) fruit ripening in early- and mid-season varieties. Plants 9:1796
Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E (2004) The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J 23(7):1647–1656. https://doi.org/10.1038/sj.emboj.7600121
Leng P, Yuan B, Guo Y (2014) The role of abscisic acid in fruit ripening and responses to abiotic stress. J Exp Bot 65(16):4577–4588. https://doi.org/10.1093/jxb/eru204
Li C, Jia H, Chai Y, Shen Y (2011) Abscisic acid perception and signaling transduction in strawberry: a model for non-climacteric fruit ripening. Plant Signaling Behav 6(12):1950–1953. https://doi.org/10.4161/psb.6.12.18024
Li Q, Chen P, Dai S, Sun Y, Yuan B, Kai W, Pei Y, He S, Liang B, Zhang Y, Leng P (2015) PacCYP707A2 negatively regulates cherry fruit ripening while PacCYP707A1 mediates drought tolerance. J Exp Bot 66(13):3765–3774. https://doi.org/10.1093/jxb/erv169
Liang D, Zhu T, Deng Q, Lin L, Tang Y, Wang J, Wang X, Luo X, Zhang H, Lv X, Xia H (2020) PacCOP1 negatively regulates anthocyanin biosynthesis in sweet cherry (Prunus avium L.). J Photochem Photobiol B Biol 203:111779. https://doi.org/10.1016/j.jphotobiol.2020.111779
Liu Y, Shen X, Zhao K, Ben Y, Guo X, Zhang X, Li T (2013) Expression analysis of anthocyanin biosynthetic genes in different colored sweet cherries (Prunus avium L.) during fruit development. J Plant Growth Regul 32(4):901–907. https://doi.org/10.1007/s00344-013-9355-3
Luo H, Dai S, Ren J, Zhang C, Ding Y, Li Z, Sun Y, Ji K, Wang Y, Li Q, Chen P, Duan C, Wang Y, Leng P (2014) The role of ABA in the maturation and postharvest life of a nonclimacteric sweet cherry fruit. J. Plant Growth Regul 33(2):373–383. https://doi.org/10.1007/s00344-013-9388-7
McAtee P, Karim S, Schaffer R, David K (2013) A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front Plant Sci 4:79. https://doi.org/10.3389/fpls.2013.00079
Meisel L, Fonseca B, Gonzalez S, Baeza-Yates R, Cambiazo V, Campos R, Gonzalez M, Orellana A, Retamales J, Silva H (2005) A rapid and efficient method for purifying high quality total RNA from peaches (Prunus persica) for functional genomics analyses. Biol Res 38(1):83–88. https://doi.org/10.4067/s0716-97602005000100010
Michailidis M, Karagiannis E, Tanou G, Samiotaki M, Tsiolas G, Sarrou E, Stamatakis G, Ganopoulos I, Martens S, Argiriou A, Molassiotis A (2020) Novel insights into the calcium action in cherry fruit development revealed by high-throughput mapping. Plant Mol Biol 104:597–614. https://doi.org/10.1007/s11103-020-01063-2
Nagpala EGL, Noferini M, Farneti B, Piccinini L, Costa G (2017) Cherry-Meter: an innovative non-destructive (vis/NIR) device for cherry fruit ripening and quality assessment. Acta Hortic 1161:491–496. https://doi.org/10.17660/ActaHortic.2017.1161.78
Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TFF, Alfred SE, Bonetta D, Finkelstein R, Provart NJ, Desveaux D, Rodriguez PL, McCourt P, Zhu JK, Schroeder JI, Volkman BF, Cutler SR (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324(5930):1068–1071. https://doi.org/10.1126/science.1173041
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):45–445. https://doi.org/10.1093/nar/29.9.e45
Ren J, Sun L, Wu J, Zhao S, Wang C, Wang Y, Ji K, Leng P (2010) Cloning and expression analysis of cDNAs for ABA 8′-hydroxylase during sweet cherry fruit maturation and under stress conditions. J Plant Physiol 167(17):1486–1493. https://doi.org/10.1016/j.jplph.2010.05.027
Ren J, Chen P, Dai SJ, Li P, Li Q, Ji K, Wang YP, Leng P (2011) Role of abscisic acid and ethylene in sweet cherry fruit maturation: molecular aspects. NZJ Crop Hortic Sci 39(3):161–174. https://doi.org/10.1080/01140671.2011.563424
Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37(6):45. https://doi.org/10.1093/nar/gkp045
Seo M, Jikumaru Y, Kamiya Y. (2011) Profiling of hormones and related metabolites in seed dormancy and germination studies. Seed Dormancy, Humana Press, 99-111. https://doi.org/10.1007/978-1-61779-231-1_7
Shen X, Zhao K, Liu L, Zhang K, Yuan H, Liao X, Wang Q, Guo X, Li F, Li T (2014) A role for PacMYBA in ABA-regulated anthocyanin biosynthesis in red-colored sweet cherry cv. Hong Deng (Prunus avium L.). Plant Cell Physiol 55(5):862–880. https://doi.org/10.1093/pcp/pcu013
Shen X, Guo X, Zhao D, Zhang Q, Jiang Y, Wang Y, Peng X, Wei Y, Zhai Z, Zhao W, Li T (2017) Cloning and expression profiling of the PacSnRK2 and PacPP2C gene families during fruit development, ABA treatment, and dehydration stress in sweet cherry. Plant Physiol Biochem 119:275–285. https://doi.org/10.1016/j.plaphy.2017.08.025
Soto A, Ruiz KB, Ravaglia D, Costa G, Torrigiani P (2013) ABA may promote or delay peach fruit ripening through modulation of ripening- and hormone-related gene expression depending on the developmental stage. Plant Physiol Biochem 64:11–24. https://doi.org/10.1016/j.plaphy.2012.12.011
Starkevič P, Paukštytė J, Kazanavičiūtė V, Denkovskienė E, Stanys V, Bendokas V, Šikšnianas T, Ražanskienė A, Ražanskas R (2015) Expression and anthocyanin biosynthesis-modulating potential of sweet cherry (Prunus avium L.) MYB10 and bHLH genes. PloS one 10(5):e0126991. https://doi.org/10.1371/journal.pone.0126991
Sutthiwal S (2012) Roles of abscisic acid in fruit ripening. Walailak J Sci Tech 9(4):297–308
Teribia N, Tijero V, Munne-Bosch S (2016) Linking hormonal profiles with variations in sugar and anthocyanin contents during the natural development and ripening of sweet cherries. New Biotechnol 33(6):824–833. https://doi.org/10.1016/j.nbt.2016.07.015
Udvardi MK, Czechowski T, Scheible WR (2008) Eleven golden rules of quantitative RT-PCR. The Plant cell 20(7):1736–1737. https://doi.org/10.1105/tpc.108.061143
Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci U S A 106(41):17588–17593. https://doi.org/10.1073/pnas.0907095106
Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K (2010) Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant Cell Physiol 51(11):1821–1839. https://doi.org/10.1093/pcp/pcq156
Wang Y, Chen P, Sun L, Li Q, Dai S, Sun Y, Kai W, Zhang Y, Liang B, Leng P (2015) Transcriptional regulation of PaPYLs, PaPP2Cs and PaSnRK2s during sweet cherry fruit development and in response to abscisic acid and auxin at onset of fruit ripening. Plant Growth Regul 75(2):455–464. https://doi.org/10.1007/s10725-014-0006-x
Wheeler S, Loveys B, Ford C, Davies C (2009) The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aust J Grape Wine Res 15(3):195–204. https://doi.org/10.1111/j.1755-0238.2008.00045.x
Zhang M, Yuan B, Leng P (2009) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60(6):1579–1588. https://doi.org/10.1093/jxb/erp026
Acknowledgements
We would like to thank Natalia Molina for her technical assistance in the field during sweet cherry fruit development and at harvest.
Availability of data and material
All relevant data are within the manuscript and its Supporting Information files.
Code availability
Not applicable
Funding
This study was funded through the project CONICYT FONDECYT/Regular No. 1171016 in collaboration with the project CONICYT FONDECYT/Regular No. 1161377. Nathalie Kuhn was supported by CONICYT FONDECYT de Postdoctorado 2018, No. 3180138. Alson Time was supported by Conicyt Scholarship Grant No. 21190238.
Author information
Authors and Affiliations
Contributions
Nathalie Kuhn: conceptualization, methodology, investigation, visualization, writing–original draft. Claudio Ponce: conceptualization, methodology, investigation, visualization, writing–original draft. Macarena Arellano: investigation, writing–review and editing. Alson Time: Investigation, writing–review and editing. Salvatore Multari: investigation, writing–review and editing. Stefan Martens: investigation, supervision, writing–review and editing. Ester Carrera: investigation, writing–review and editing. Boris Sagredo: conceptualization, writing–review and editing. José Manuel Donoso: conceptualization, writing–review and editing. Lee A. Meisel: conceptualization, methodology, visualization, supervision, project administration, funding acquisition, writing–review and editing
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Data Archiving Statement
All relevant data are within the manuscript and its Supporting Information files.
Additional information
Communicated by C. Dardick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Communicated by C. Dardick
Supplementary information
Fig. S1
Schematic representation of the fruit sampling for quality parameters measurement at harvest and the experimental design of the ABA treatment. (a), Twenty-five fruits of (n=4) trees were collected on November 29th, 2017 (1st harvest; 69 DAFB), and December 1st , 2017 (2nd harvest; 75 DAFB) for the analysis at harvest. 25 and 15 fruits per tree (n=4 trees) were used for quantifying the parameters in the 1st and 2nd harvest, respectively. The five most homogeneous fruits among the 25 or 15 were used for SSC (soluble solid content) and acidity determination. A. Refractometer; B. Durometer; C. Cherry meter; D. CTIFL color chart for color distribution; E. Fruit Caliper; F. Scale. (b), Diagram showing the number of trees treated with ABA and the control trees. Additionally, a picture of representative fruits minutes prior the ABA treatment (56 DAFB; November 16th, 2017; P1) is included. (PNG 1583 kb)
Fig. S2
Effect of ABA on ripening related parameters. (a), Fruit growth curve of ABA and control trees. The arrows indicate ABA treatment at 56 DAFB, as well as the 1st and 2nd fruit harvests. The asterisks indicate statistical differences at the indicated dates determined with T-student test, p-value < 0.05. (b), Percentage change of fruit quality parameters (width, weight, firmness, soluble solids, and acidity) in response to ABA treatment at 1st and 2nd harvest. (c), Representative pictures of the control and ABA-treated fruits at 2nd harvest. (PNG 820 kb)
Fig. S3
Transcript abundance relative to PavCAC of putative orthologs of ABA signaling pathway during fruit development. (a), PavPYL2 gene, (b), PavCYP707A1 gene, (c), PavMYB gene, encoding putative R2R3 MYB transcription factor . LG, P1, P2, and R are Light green, Pink 1, Pink 2, and Red stages, respectively (see Table S2). Pools of eight fruits per tree (n=3 trees) were used. Data were represented as mean ± SEM. (PNG 587 kb)
Fig. S4
Effect of ABA treatment on the transcript abundance relative to PavCAC of putative orthologs of the ABA signaling pathway. (a), PavPYL2 gene, (b), PavCYP707A1 gene, and (c), PavMYBA gene, encoding putative R2R3 MYB transcription factor. Pools of eight fruits per tree (n=3 trees) for control and ABA-treated trees were used. Data were represented as mean ± SEM and the values were set to 1.0 in Control. (PNG 200 kb)
ESM 5
(DOCX 14 kb)
ESM 6
(DOCX 14 kb)
ESM 7
(DOCX 14 kb)
ESM 8
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Kuhn, N., Ponce, C., Arellano, M. et al. ABA influences color initiation timing in P. avium L. fruits by sequentially modulating the transcript levels of ABA and anthocyanin-related genes. Tree Genetics & Genomes 17, 20 (2021). https://doi.org/10.1007/s11295-021-01502-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-021-01502-1