Abstract
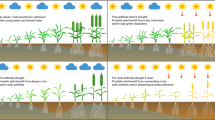
Maritime pine grows naturally under a wide range of climatic conditions, from strongly Atlantic to strongly Mediterranean. Aiming to improve our understanding of the genetic structure and inheritance of drought resistance strategies in the species, we conducted an environmentally controlled experiment to assess the genetic variation and plasticity to drought of Atlantic and Mediterranean populations, and the interprovenance hybrids between them. Hybridization could also help to provide new genetic material for use in transitional areas between the two regions, for which reproductive materials of good quality are generally lacking. Plastic responses to water stress appeared to be highly conserved among populations, with a common conservative isohydric strategy based on promoting growth when water was abundant, and stopping it when water became limiting. We found, however, a strong intraspecific variation in biomass allocation patterns. The Atlantic populations showed a risky growth-based strategy with a larger amount of juvenile needles, whereas Mediterranean populations showed a more conservative strategy, minimizing aerial growth and increasing the proportion of adult needles that is more resistant to water loss. Hybrid populations performed more similarly to the Mediterranean parent, suggesting a dominance of the Mediterranean-like characteristics. Some of the tested hybrid populations, however, combined high growth with traits of drought adaptation, and thus represent potentially interesting materials for use in transitional regions between the two climate zones.




Similar content being viewed by others
References
Alberto FJ, Aitken SN, Alía R, González-Martínez SC, Häninen H et al (2013) Potential for evolutionary responses to climate change—evidence from tree populations. Glob Chang Biol 19:1645–1661
Aranda I, Alía R, Ortega U, Dantas AK, Majada J (2010) Intra-specific variability in biomass partitioning and carbon isotopic discrimination under moderate drought stress in seedlings from four Pinus pinaster populations. Tree Genet Genomes 6:169–178
Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitation in plants—an economic analogy. Annu Rev Ecol Syst 16:363–392
Bolstad SB, Kang H, Guries RP, Marty TL (1991) Performance of interprovenance and intraprovenance crosses of jack pine in Central Wisconsin. Silvae Genet 40:124–130
Cendán C, Sampedro L, Zas R (2013) The maternal environment determines the timing of germination in Pinus pinaster. Env Exp Bot 94:66–72
Chambel MR, Climent J, Alía R (2007) Divergence among species and populations of Mediterranean pines in biomass allocation of seedlings grown under two watering regimes. Ann For Sci 64:87–97
Climent J, Silva FCE, Chambel MR, Pardos M, Almeida MH (2009) Freezing injury in primary and secondary needles of Mediterranean pine species of contrasting ecological niches. Ann For Sci 66:407
Cody ML (1986) Roots in plant ecology. Trends Ecol Evol 1:76–78
Coleman JS, McConnaughay KDM, Ackerly DD (1994) Interpreting phenotypic variation in plants. Trends Ecol Evol 9:187–191
Corcuera L, Gil-Pelegrín E, Notivol E (2010) Phenotypic plasticity in Pinus pinaster delta(13)C: environment modulates genetic variation. Ann For Sci 67:812
Corcuera L, Cochard H, Gil-Pelegrín E, Notivol E (2011) Phenotypic plasticity in mesic populations of Pinus pinaster improves resistance ti xylem embolism (P50) under severe drought. Trees 25:1033–1042
Corcuera L, Gil-Pelegrín E, Notivol E (2012) Differences in hydraulic architecture between mesic and xeric Pinus pinaster populations at the seedling stage. Tree Physiol 32:1442–1457
Correia I, Almeida MH, Aguiar A, Alía R, David TS, Pereira JS (2008) Variations in growth, survival and carbon isotope composition (13C) among Pinus pinaster populations of different geographic origins. Tree Physiol 28:1545–1552
Danjon F, González G, Meredieu C et al (2009) Phenotypic plasticity of Pinus pinaster to water stress: biomass allocation and root architecture. In: International Symposium “Root Research and Applications”, RootRAP, Boku, Vienna, Austria, 2–4 September
Darrow HE, Bannister P, Burritt DJ, Jameson PE (2002) Are juvenile forms of New Zealand heteroblastic trees more resistant to water loss than their mature counterparts? N Z J Bot 40:313–325
de la Mata R, Zas R (2010a) Performance of maritime pine Spanish Mediterranean provenances at young ages in a transitional region between Atlantic and Mediterranean climates in NW Spain. Silvae Genet 59:8–17
de la Mata R, Zas R (2010b) Transferring Atlantic maritime pine improved material to a region with marked Mediterranean influence in inland NW Spain: a likelihood-base approach on spatially adjusted field data. Eur J For Res 129:645–658
Eriksson G, Ilstedt B (1986) Stem volume of intra- and interprovenance families of Picea abies (L.) Karst. Scand J For Res 1:141–152
Fernández M, Gil L, Pardos JA (1999) Response of Pinus pinaster Ait. provenances at early age to water supply. I. Water relation parameters. Ann For Sci 56:179–187
Fernández M, Gil L, Pardos JA (2000) Effects of water supply on gas exchange in Pinus pinaster Ait. provenances during their first growing season. Ann For Sci 57:9–16
Gaspar MJ, Velasco T, Feito I, Alía R, Majada J (2013) Genetic variation of drought tolerance in Pinus pinaster at three hierarchical levels: a comparison of induced osmotic stress and field testing. PLoS ONE 8(11):e79094
González-Martínez SC, Mariette S, Ribeiro MM, Burban C et al (2004) Genetic resources in maritime pine (Pinus pinaster Aiton): molecular and quantitative measures of genetic variation and differentiation among maternal lineages. For Ecol Manag 197:103–115
Grotkopp E, Rejmánek M, Rost TL (2002) Toward a causal explanation of plant invasiveness: seedling growth and life-history strategies of 29 pine (Pinus) species. Am Nat 159(4):396–419
Harfouche A, Kremer A (2000) Provenance hybridization in a diallel mating scheme of maritime pine (Pinus pinaster). I. Means and variance components. Can J For Res 30:1–9
Harfouche A, Baradat P, Kremer A (1995) Variabilité intraspécifique chez le pin maritime (Pinus pinaster Ait) dans le sud-est de la France. II. Hétérosis et combinaison de caractères chez des hybrides interraciaux. Ann For Sci 52:329–346
IPCC (2007) Intergovernmental Panel on Climate Change, Fourth Assessment Report. In: Pachauri R, Reisinger A (eds). Geneva, Switzerland, p 104
Kaya Z, Lindgren D (1992) The genetic variation of inter-provenance hybrids of Picea abies and possible breeding consequences. Scand J For Res 7:15–26
Lamy JB, Delzon S, Bouche P, Alía R, Vendramin GG et al (2014) Limited genetic variability and phenotypic plasticity detected for cavitation resistance in a Mediterranean pine. New Phytol 201:874–886
Larcher W (1995) Physiological plant ecology. Ecophysiology and stress physiology of functional groups. Springer, Berlin-Heidelberg
Leck MA, Parker VT, Simpson RL (2008) Seedling ecology and evolution. Cambridge University Press, Cambridge
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS System for mixed models, 2nd edn. SAS Institute, Cary
Ludlow MM (1989) Strategies of response to water stress. In: Kreeb KH, Richter H, Hinckley TM (eds) Structural and functional responses to environmental stresses: water shortage. SPB Academic Publishing, The Hague, pp 269–281
Martínez-Ferri E, Balaguer L, Valladares F, Chico JM, Manrique E (2000) Energy dissipation in drought-avoiding and drought-tolerant tree species at midday during the Mediterranean summer. Tree Physiol 20:131–138
Müller I, Schmid B, Weiner J (2000) The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspect Plant Ecol 3:115–127
Nguyen A, Lamant A (1989) Variation in growth and osmotic regulation of roots of water-stressed maritime pine (Pinus pinaster Ait.) provenances. Tree Physiol 5:123–133
Nguyen-Queyrens A, Costa P, Loustau D, Plomion C (2002) Osmotic adjustment in Pinus pinaster cuttings in response to a soil drying cycle. Ann For Sci 59:795–799
Niklas KJ (1994) Allometry in plants: the scaling of form and process. University of Chicago Press, Chicago
O’Brien EK, Mazanec RA, Krauss SL (2007) Provenance variation of ecologically important traits of forest trees: implications for restoration. J Appl Ecol 44:583–593
Osório J, Osório ML, Chaves MM, Pereira JS (1998) Water deficits are more important in delaying growth than in changing patterns of carbon allocation in Eucalyptus globulus. Tree Physiol 18:363–373
Pardos M, Calama R, Climent J (2009) Difference in cuticular transpiration and sclerophylly in juvenile and adult pine needles relates to the species-specific rates of development. Trees Struct Funct 23:501–508
Peters J, Morales D, Jiménez MS (2003) Gas exchange characteristics of Pinus canariensis needles in a forest stand on Tenerife, Canary Islands. Trees Struct Funct 17:492–500
Pigliucci M, Schlichting CD (1996) Reaction norms of Arabidopsis. IV. Relationships between plasticity and fitness. Heredity 76:427–436
Pigott CD, Pigott S (1993) Water as a determinant of the distribution of trees at the boundary of the Mediterranean Zone. J Ecol 81:557–566
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol 27:595–607
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50
Sánchez-Gómez D, Majada J, Alía R, Feito I, Aranda I (2010) Intraspecific variation in growth and allocation patterns in seedlings of Pinus pinaster Ait. submitted to contrasting watering regimes: can water availability explain regional variation? Ann For Sci 67:1–8
Shukla RP, Ramakrishnan PS (1986) Architecture and growth strategies of tropical trees in relation to successional status. J Ecol 74:33–46
Strauss SH, Ledig FT (1985) Seedling architecture and life history evolution in pines. Am Nat 125:702–715
Tognetti R, Michelozzi M, Lauteri M, Brugnoli E, Giannini R (2000) Geographic variation in growth, carbon isotope discrimination, and monoterpene composition in Pinus pinaster Ait. provenances. Can J For Res 30:1682–1690
White TL, Adams WT, Neale DB (2007) Forest genetics. CAB International, Wallingford
Acknowledgments
We gratefully acknowledge the effort made by many researchers and technicians of the Lourizán Forestry Research Centre, who undertook the controlled pollination, seeded the donor plants, propagated the plant material, installed the trial, applied the treatments, and assessed the traits. Particularly, we thank Sara Varela, Ana Hernández, Luis Sampedro, Xoaquín Moreira, José María Mendaña, Marisa Blanco, Maribel Juncal, Manuel Cerviño, and Emilio Pérez. We are also grateful to R. García Arranz for pollen collection in Espinoso del Rey. We acknowledge D. Brown for language revision. Valuable comments by three anonymous reviewers also helped to improve the quality of the manuscript. This study was partially supported by the research grants RTA07-100, PSE310000, and AGL2010-18724-COMPROPIN. RM is undertaking a postdoctoral scholarship at The University of Montana funded by the Barrié Foundation.
Data Archiving
Data has been submitted to the repository of the Spanish National Research Council (Digital.CSIC, http://digital.csic.es/) Accession number will be supplied once available.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. N. Aitken
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(PDF 138 kb)
Online Resource 2
(PDF 1952 kb)
Online Resource 3
(PDF 108 kb)
Rights and permissions
About this article
Cite this article
de la Mata, R., Merlo, E. & Zas, R. Among-population variation and plasticity to drought of Atlantic, Mediterranean, and interprovenance hybrid populations of maritime pine. Tree Genetics & Genomes 10, 1191–1203 (2014). https://doi.org/10.1007/s11295-014-0753-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11295-014-0753-x