Abstract
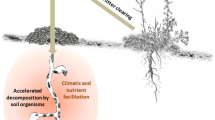
Ungulates use habitat with differential microclimate characteristics; therefore, fecal inputs to a particular habitat may result in vastly different rates of decomposition and nutrient release. We tested this hypothesis and conducted a 1-year decomposition experiment where we deployed fecal samples from Shiras moose (Alces alces shirasi) and subsequently measured loss of fecal mass, nutrient release captured with resin bags, and associated consequences for nitrogen (N) cycling in soils. The microhabitat type in which fecal samples were deployed influenced rates of decomposition observed; samples experienced faster rates of decomposition in a riparian habitat type than a conifer site. Cumulative nutrient losses as nitrate (NO3 −) measured with anion and cation exchange resin bags were significantly higher in the conifer site (0.08 g N/feces) than the riparian site (0.02 g N/feces), whereas ammonium (NH4 +) losses, though higher than nitrate losses, were not significantly different between the riparian site (0.40 g N/feces) and the conifer site (0.26 g N/feces) after 1-year. Concentrations of soil NH4 + and NO3 − beneath the fecal samples in the riparian site were significantly higher relative to control soils after 1 year, but no differences were detected in the conifer site. Cumulatively, our findings supported our hypothesis that fecal deposition by large herbivores can strongly influence nutrient releases to the ecosystem through the decomposition of fecal matter. Such nutrient additions may have direct effects on N cycling in soil and provide valuable inputs that are available for plant uptake and subsequent growth.



Similar content being viewed by others
References
Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, Hall SA, Burkes IC, Hart SC (2008) Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob Change Biol 14:2636–2660
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Bardgett RD, Wardle D, Yeates G (1998) Linking above-ground and below-ground interactions: how plant responses to foliar herbivory influence soil organisms. Soil Biol Biochem 30:1867–1878
Bowyer RT, Van Ballenberghe V, Kie JG (1997) The role of moose in landscape processes: effects of biogeography, population dynamics, and predation. In: Bissonette JA (ed) Wildlife and landscape ecology: effects and patterns of scale. Springer, New York, pp 265–287
Bowyer RT, Bleich VC, Manteca X, Whiting JC, Stewart KM (2007) Sociality, mate choice, and timing of mating in American bison (Bison bison): effects of large males. Ethology 113:1048–1060
Butler LG, Kielland K (2008) Acceleration of vegetation turnover and element cycling by mammalian herbivory in riparian ecosystems. J Ecol 96:136–144
Campbell D, Swanson GM, Sales J (2004) Comparing the precision and effectiveness of faecal pellet group count methods. J Appl Ecol 41:1185–1196
Chapin FS III, Matson PA, Mooney HA (2011) Principles of terrestrial ecosystem ecology, 2nd edn. Springer, New York
Christenson LM, Mitchell MJ, Groffman PM, Lovett GM (2010) Winter climate change implications for decomposition in northeastern forests: comparisons of sugar maple litter with herbivore fecal inputs. Glob Change Biol 16:2589–2601
Cornelissen JHC (1996) An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J Ecol 84:573–582
Day TA, Detling JK (1990) Grassland patch dynamics and herbivore grazing preference following urine depositions. Ecology 71:180–188
Dussault C, Ouellet JP, Courtois R, Huot J, Breton L, Jolicoeur H (2005) Linking moose habitat selection to limiting factors. Ecography 28:619–628
Frank DA, Groffman PM (1998) Ungulates versus topographic control of soil carbon and nitrogen processes in grasslands of Yellowstone National Park. Ecology 79:2229–2241
Harmon ME, Nadelhoffer KJ, Blair JM (1999) Measuring decomposition, nutrient turnover, and stores in plant litter. In: Robertson GP, Coleman DC, Bledsoe CS, Sollings P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 202–240
Hobbs NT (1996) Modification of ecosystems by ungulates. J Wildl Manage 60:695–713
Jenks JA, Soper RB, Lochmiller RL, Leslie DM Jr (1990) Effects of exposure on nitrogen and fiber characteristics of white-tailed deer feces. J Wildl Manage 54:389–391
Köchy M, Wilson SD (1997) Litter decomposition and nitrogen dynamics in aspen forest and mixed-grass prairie. Ecology 78:732–739
Lohse KA, Matson P (2005) Consequences of nitrogen additions for soil processes and solution losses from wet tropical forests. Ecol Appl 15:1629–1648
Maier JAK, Ver Hoef JM, McGuire AD, Bowyer RT, Saperstein L, Maier HA (2005) Distribution and density of moose in relation to landscape characteristics: effects of scale. Can J For Res 35:2233–2243
Masunga GS, Andresen O, Taylor J, Dhillion S (2006) Elephant dung decomposition and coprophilous fungi in two habitats of semi-arid Botswana. Mycol Res 110:1214–1226
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Menezes RSC, Elliott ET, Valentine DW, Williams SA (2001) Carbon and nitrogen dynamics in elk winter ranges. J Range Manage 54:400–408
Molvar EM, Bowyer RT, Van Ballenberghe V (1993) Moose herbivory, browse quality, and nutrient cycling in an Alaskan treeline community. Oecologia 94:472–479
Monteith KB, Monteith KL, Bowyer RT, Leslie DM Jr, Jenks JA (2014) Reproductive effects on fecal nitrogen as an index to diet quality: an experimental assessment. J Mammal 95:301–310
Moore TR, Trofymow JA, Prescott CE, Fyles J, Titus BD (2006) Patterns of carbon, nitrogen and phosphorus dynamics in decomposing foliar litter in Canadian forests. Ecosystems 9:46–62
Oehlers SA, Bowyer RT, Huettman F, Person DK, Kessler WB (2011) Sex and scale: implications for habitat selection by Alaskan moose Alces alces gigas. Wildl Biol 17:67–84
Olofsson J, Oksanen L (2002) Role of litter decomposition for the increased primary production in areas heavily grazed by reindeer: a litterbag experiment. Oikos 96:507–515
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC, Fasth B (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364
Pastor J, Naiman RJ (1992) Selective foraging and ecosystem processes in boreal forests. Am Nat 139:690–705
Pastor J, Dewey B, Naiman RJ, McInnes P, Cohen Y (1993) Moose browsing and soil fertility in the boreal forests of Isle Royale National Park. Ecology 74:467–480
Persson IL, Danell J, Bergstrom R (2000) Disturbance by large herbivores in boreal forests with special reference to moose. Ann Zool Fennici 37:251–263
Renecker LA, Hudson RJ (1988) Seasonal quality of forages used by moose in the aspen dominated boreal forest, central Alberta. Holarct Ecol 11:111–118
Risch AC, Jurgensen MF, Frank DA (2007) Effects of grazing and soil micro-climate on decomposition rates in a spatio-temporally heterogeneous grassland. Plant Soil 298:191–201
Risenhoover KL, Maass SA (1987) The influence of moose on the composition and structure of Isle Royale forests. Can J For Res 17:357–364
Robertson GP, Paul EA (2000) Decomposition and soil organic matter dynamics. In: Osvaldo ES, Jackson RB, Mooney HA, Howarth R (eds) Methods of ecosystem science. Springer, New York, pp 104–116
Ruess RW, McNaughton SJ (1987) Grazing and the dynamics of nutrient and energy regulated microbial processes in the Serengeti grasslands. Oikos 49:101–110
Schoenecker KA, Singer FJ, Zeigenfuss LC, Binkley D, Menezes RSC (2004) Effects of elk herbivory on vegetation and nitrogen processes. J Wildl Manage 68:837–849
Stewart KM, Bowyer RT, Kie JG, Dick BL, Ruess RW (2009) Population density of North American elk: effects on plant diversity. Oecologia 161:303–312
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. University of California Press, Berkeley
Taylor BR (1998) Air-drying depresses rates of leaf litter decomposition. Soil Biol Biochem 30:403–412
Throop HL, Archer SR (2007) Interrelationships among shrub encroachment, land management, and litter decomposition in a semidesert grassland. Ecol Appl 17:1809–1823
Vitousek P (1982) Nutrient cycling and nutrient use efficiency. Am Nat 119:553–572
Walter H, Lieth H (1967) Klimadiagramm-Weltatlas. Gustav Fischer Verlag, Stuttgart
Wardle DA, Bardgett RD (2004) Human-induced changes in large herbivorous mammal density: the consequences for decomposers. Front Ecol Environ 2:145–153
Yavitt JB, Fahey TJ (1986) Litter decay and leaching from the forest floor in Pinus contorta (lodgepole pine) ecosystems. J Ecol 74:525–545
Acknowledgments
We thank P. Lendrum, S. Prestigiacomo, M. Franco, and L. Waters for their assistance in the field and laboratory. In addition, we thank M. Kauffman and P. Lendrum who reviewed earlier versions of this manuscript and provided valuable comments. Financial support for this project was provided by the Idaho State University Research Committee and the Geological Society of America. K. Lohse and N. Guernsey were supported by NSF Idaho EPSCoR (EPS-0814387).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Guernsey, N.C., Lohse, K.A. & Bowyer, R.T. Rates of decomposition and nutrient release of herbivore inputs are driven by habitat microsite characteristics. Ecol Res 30, 951–961 (2015). https://doi.org/10.1007/s11284-015-1296-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-015-1296-9