Abstract
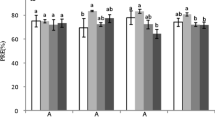
Nutrient resorption from senescing leaves is a key nutrient conservation strategy in many ecosystems. In evergreen broad-leaved forests, nutrient resorption occurs with concomitant defoliation almost year-round. However, it is unclear whether nutrient resorption efficiency (NuRE) varies in different defoliation seasons for evergreen broad-leaved trees and how it may relate to soil nutrient availability. Castanopsis eyrei is a dominant tree species in evergreen broad-leaved forests in southern China, with distinct bimodal patterns of defoliation in spring and autumn. We investigated seasonal variation of the resorption efficiency of nitrogen (N) and phosphorus (P) of C. eyrei leaf and its relationship with soil mineral N by measuring the concentrations in leaf, leaf litter, and soil for two consecutive years. We hypothesized that leaf NuRE should be higher in seasons with low soil nutrient availability. We found that on average, soil mineral N content was 38.5 % lower during the autumn defoliation peak than in spring, and N and P concentrations in leaf litter at the spring defoliation peak were 10.0 and 26.7 % higher than at the autumn peak. Nitrogen NuRE at the autumnal defoliation peak was 5.1 % higher than that at the spring peak (49.3 vs 44.2 %), and the phosphorus NuRE in autumn was 6.8 % higher than that in spring (77.6 vs 69.8 %). Our findings suggest an inverse relationship between soil nutrient availability and NuRE and that the dynamics in NuRE should be incorporated into modeling of biogeochemical cycling in evergreen forest ecosystems with a bimodal defoliation pattern.





Similar content being viewed by others
References
Addicott F (1991) Abscission: shedding of parts. Physiology of trees. Wiley, New York, pp 273–300
Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol 84:597–608
Aerts R, Chapin F III (2000) The mineral nutrition of wild plants revisited. Adv Ecol Res 30:1–67
Anderson J, Ingram J (1994) Tropical soil biology and fertility: a handbook of methods. Soil Sci 157:265
Barlow J, Gardner TA, Ferreira LV, Peres CA (2007) Litter fall and decomposition in primary, secondary and plantation forests in the Brazilian Amazon. For Ecol Manage 247:91–97
Bausenwein U, Millard P, Thornton B, Raven J (2001) Seasonal nitrogen storage and remobilization in the forb Rumex acetosa. Funct Ecol 15:370–377
Berg B (2000) Litter decomposition and organic matter turnover in northern forest soils. For Ecol Manage 133:13–22
Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitation in plants—an economic analogy. Annu Rev Ecol Syst 16:363–392
Bray JR, Gorham E (1964) Litter production in forests of the world. Adv Ecol Res 2:101–157
Cassman K, Munns D (1980) Nitrogen mineralization as affected by soil moisture, temperature, and depth. Soil Sci Soc Am J 44:1233–1237
Chapin FS III, Kedrowski RA (1983) Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 64:376–391
Chapin FS III, Moilanen L (1991) Nutritional controls over nitrogen and phosphorus resorption from Alaskan birch leaves. Ecology 72:709–715
Chapin FS III, Johnson DA, McKendrick JD (1980) Seasonal movement of nutrients in plants of differing growth form in an Alaskan tundra ecosystem: implications for herbivory. J Ecol 68:189–209
Chapin FS, Schulze E-D, Mooney HA (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21:423–447
Chaudhry S (2005) Nitrogen resorption in leaves of tree and shrub seedlings in response to increasing soil fertility. Curr Sci 89:389–396
Constantinides M, Fownes J (1994) Nitrogen mineralization from leaves and litter of tropical plants: relationship to nitrogen, lignin and soluble polyphenol concentrations. Soil Biol Biochem 26:49–55
Del Arco JM, Escudero A, Garrido MV (1991) Effects of site characteristics on nitrogen retranslocation from senescing leaves. Ecology 72:701–708
Dickson R (1989) Carbon and nitrogen allocation in trees. In: Annales des sciences forestières, vol 46, no Supplement. EDP Sciences, pp 631s–647s
Du E, Zhou Z, Li P, Hu X, Ma Y, Wang W, Zheng C, Zhu J, He J, Fang J (2013) NEECF: a project of nutrient enrichment experiments in china’s forests. J Plant Ecol. doi:10.1093/jpe/rtt008
Duan W, Zheng W, Yan W, Liang X, Li S (2012) Seasonal dynamic of nitrogen mineralization in soils of Cinnamomum camphora and Pinus massoniana plantations. J Cent South Univ For Technol 31:96–100 (In Chinese with English Abstract)
Eckstein R, Karlsson P, Weih M (1999) Leaf life span and nutrient resorption as determinants of plant nutrient conservation in temperate-arctic regions. New Phytol 143:177–189
Fernandez-Escobar R, Moreno R, Garcıa-Creus M (1999) Seasonal changes of mineral nutrients in olive leaves during the alternate-bearing cycle. Sci Hortic 82:25–45
Han YL (1990) Scientific survey of the Guniujiang natural reserve. China Expectation Press, Beijing
Han W, Tang L, Chen Y, Fang J (2013) Relationship between the Relative Limitation and Resorption Efficiency of Nitrogen vs Phosphorus in Woody Plants. PLoS One 8:e83366
Hart SC, Nason GE, Myrold DD, Perry DA (1994) Dynamics of gross nitrogen transformations in an old-growth forest: the carbon connection. Ecology 75:880–891
Jarvis P, Leverenz J (1983) Productivity of temperate, deciduous and evergreen forests. In: Physiological plant ecology IV. Springer, pp 233–280
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77:1716–1727
Kira T, Ono Y, Hosokawa T (1978) Biological production in a warm-temperate evergreen oak forest of Japan. University of Tokyo Press, Tokyo
Kirschbaum MU (1995) The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biol Biochem 27:753–760
Kobe RK, Lepczyk CA, Iyer M (2005) Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 86:2780–2792
Lajtha K (1987) Nutrient reabsorption efficiency and the response to phosphorus fertilization in the desert shrub Larrea tridentata (DC.) Cov. Biogeochemistry 4:265–276
Lambers H, Chapin IFS, Chapin FS, Pons TL (2008) Plant physiological ecology. Springer, New York, pp 302–328
Li J, Sha L, Wang J, Feng W, Chen J, Li J (2006) Seasonal variation of soil nitrogen mineralization in a montane moist evergreen broad-leaved forest in Ailao Mountains, SW China. J Mt Sci 24:186–192 (In Chinese with English Abstract)
Li X, Zheng X, Han S, Zheng J, Li T (2010) Effects of nitrogen additions on nitrogen resorption and use efficiencies and foliar litterfall of six tree species in a mixed birch and poplar forest, northeastern China. Can J For Res 40:2256–2261
Lowman MD (1992) Leaf growth dynamics and herbivory in five species of Australian rain-forest canopy trees. J Ecol 80:433–447
Matthews E (1997) Global litter production, pools, and turnover times: estimates from measurement data and regression models. J Geophys Res 102:18771–18800
Meentemeyer V, Box EO, Thompson R (1982) World patterns and amounts of terrestrial plant litter production. Bioscience 32:125–128
Minoletti ML, Boerner RE (1993) Seasonal photosynthesis, nitrogen and phosphorus dynamics, and resorption in the wintergreen fern Polystichum acrostichoides (Michx.) Schott. Bulletin of the Torrey Botanical Club, pp 397–404
Nitta I, Ohsawa M (1997) Leaf dynamics and shoot phenology of eleven warm-temperate evergreen broad-leaved trees near their northern limit in central Japan. Plant Ecol 130:71–88
Ohsawa M (1990) An interpretation of latitudinal patterns of forest limits in south and east Asian mountains. J Ecol 78:326–339
Pugnaire FI, Chapin FS III (1993) Controls over nutrient resorption from leaves of evergreen Mediterranean species. Ecology 74:124–129
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, http://www.R-project.org. Accessed 1 Mar 2004
Reich P, Uhl C, Walters M, Ellsworth D (1991) Leaf lifespan as a determinant of leaf structure and function among 23 Amazonian tree species. Oecologia 86(1):16–24
Schlesinger WH, DeLucia EH, Billings W (1989) Nutrient-use efficiency of woody plants on contrasting soils in the western Great Basin, Nevada. Ecology 70:105–113
Schulze ED (1982) Plant life forms and their carbon, water and nutrient relations. In: Physiological plant ecology II. Springer, pp 615–676
Schulze E-D, Chapin III F (1987) Plant specialization to environments of different resource availability. In: Potentials and limitations of ecosystem analysis. Springer, pp 120–148
Shen Z, Hu Z, Zhao J, Wang H (2007) Altitudinal pattern of plant diversity on Mt. Guningjiang, Anhui, China- with a discussion on the ecological impacts of hilltop condition. J Mt Sci 25:160–168 (In Chinese with English Abstract)
Song K, Kohyama TS, Da LJ (2014) Transition patterns across an evergreen–deciduous broad-leaved forest ecotone: the effect of topographies. J Veg Sci. doi:10.1111/jvs.12156
Stanford G, Epstein E (1974) Nitrogen mineralization-water relations in soils. Soil Sci Soc Am J 38:103–107
Tang L, Han W, Chen Y, Fang J (2013) Resorption proficiency and efficiency of leaf nutrients in woody plants in eastern China. J Plant Ecol. doi:10.1093/jpe/rtt013
Tully KL, Wood TE, Schwantes AM, Lawrence D (2013) Soil nutrient availability and reproductive effort drive patterns in nutrient resorption in Pentaclethra macroloba. Ecology 94:930–940
Van Heerwaarden L, Toet S, Aerts R (2003) Current measures of nutrient resorption efficiency lead to a substantial underestimation of real resorption efficiency: facts and solutions. Oikos 101:664–669
Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82:205–220
Vitousek PM (1998) Foliar and Litter Nutrients, Nutrient Resorption, and Decomposition in Hawaiian Me t rosideros polymorpha. Ecosystems 1:401–407
Wright I, Westoby M (2003) Nutrient concentration, resorption and lifespan: leaf traits of Australian sclerophyll species. Funct Ecol 17:10–19
Xia H, Yu Q, Zhang D (1997) The soil acidity and nitrogen contents and their characterisitics of seasonal dynamic change under 3 different forests of Dinghushan Nature Reserve. Acta Ecologica Sinica 17:645–653 (In Chinese with English Abstract)
Yuan Z, Chen HY (2009) Global-scale patterns of nutrient resorption associated with latitude, temperature and precipitation. Glob Ecol Biogeogr 18:11–18
Acknowledgments
We thank Dr. Chengjun Ji, Dr. Zhiyao Tang, and Zhengbing Yan for their valuable suggestions on earlier versions of this manuscript. We also thank Xueyang Hu, Lai Jiang, Weibin Yin, Jiajia Zhou, and Yang Zhao for their kind suggestions and help with the fieldwork. We thank two anonymous reviewers whose helpful comments greatly improved this paper. This work was supported by the National Basic Research Program of China on Global Change (No. 2010CB950600) and by the National Natural Science Foundation of China (Nos. 31321061 and 31330012).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Li, P., Han, W., Zhang, C. et al. Nutrient resorption of Castanopsis eyrei varies at the defoliation peaks in spring and autumn in a subtropical forest, Anhui, China. Ecol Res 30, 111–118 (2015). https://doi.org/10.1007/s11284-014-1216-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-014-1216-4