Abstract
The goal of this study is to clarify how different aspects of plant function are coordinated developmentally for species of ring-porous versus diffuse-porous deciduous trees, comparing the timing of leaf phenology and vessel formation in twigs and stems from an ecophysiological viewpoint. Cylindrical stem cores and twigs were collected at intervals from early spring through summer from five ring-porous and five diffuse-porous species in a cool temperate forest, and leaf and vessel formation were observed simultaneously. We found that the first-formed vessels of the year were lignified in twigs around the time of leaf appearance and at or before full leaf expansion of each tree in both groups of species with flush-leaves. Vessels in stems were lignified 2 weeks before to 4 weeks after leaf appearance and before or around full leaf expansion of the tree in ring-porous species. This was significantly earlier than in diffuse-porous species, in which stem vessel lignification was 2–8 weeks after leaf appearance and at or after full leaf expansion of the tree. The timing of vessel formation in twigs compared to stems was significantly earlier in ring-porous species than in diffuse-porous species. Lignification of vessels in stems occurred within 2 weeks of lignification in the twigs of ring-porous species and 2–8 weeks after lignification in twigs of diffuse-porous species. These results indicate the order and time-lag of leaf and vessel formation. Ring-porous species showed intensive leaf/vessel production, whereas diffuse-porous species showed less intensive leaf/vessel production.
Similar content being viewed by others
Introduction
In a temperate zone with four seasons, trees begin photosynthesis and water transport in spring after winter dormancy, and so spring growth is important for trees. Temperate tree species have one of three types of wood porosity (Wheeler et al. 1989). Ring-porous species have considerably larger pores in earlywood than in latewood of the previous and the same growth ring. Diffuse-porous species have vessels of approximately the same diameter throughout the growth ring. Semi-ring-porous species are intermediate in character. The differences in the size and distribution of pores lead to differences in the efficiency of water conduction. Species with different types of ring porosity coexist throughout the temperate zone, and it is still unclear how ring porosity affects species’ adaptation to their environment. This study attempts to clarify this by examining the relationship between leaf phenology and vessel formation.
In broad-leaved deciduous species, there is a difference in the temporal relationships of leaf phenology and vessel formation in ring-porous and diffuse-porous species. In ring-porous species, stem diameter begins to increase before or at the time of leaf appearance, whereas in diffuse-porous species it begins after leaf appearance (Friesner 1942; Komiyama et al. 1987; Komiyama 1991; Maruyama et al. 1992).
At the microstructural level, the onset of cambial activity in early spring is controlled by growth hormones (Wilcox 1962; Fahn and Werker 1990). Cell division in bud bases begins just before or simultaneously with bud break in both ring-porous and diffuse-porous species (Ladefoged 1952). However, several studies (e.g., Wareing 1950; Ladefoged 1952) have shown that cambial activity in diffuse-porous species starts at the base of winter buds and then spreads slowly downward and that cell division occurs more quickly in the basipetal direction in ring-porous species than in diffuse-porous species. Therefore, cell division at the base of stems begins earlier in ring-porous species, a few days before or simultaneously with bud break, whereas diffuse-porous species do not show division at the stem base until foliage has partly or completely expanded (Priestley and Scott 1936; Ladefoged 1952). Other studies have reported that ring-porous species have overwintering cells (Tepper and Hollis 1967; Zasada and Zahner 1969; Imagawa and Ishida 1972), and that both ring- and diffuse-porous species initiate cambial cell division simultaneously after bud break, but overwintering cells in the cambial zone are enlarged prior to cell division in ring-porous species (Frankenstein et al. 2005). Thus, it is important to specify the stage of vessel formation that is being compared between ring- and diffuse-porous species.
After cell division and the subsequent enlargement of vessel elements in ring-porous species, the first vessel elements formed in the new growth ring, which are adjacent to the annual ring border, mature 1 week before or up to 3 weeks after leaf expansion (Suzuki et al. 1996). Vessel maturation also occurs around the time of leaf budding (Suzuki et al. 2000). In contrast, vessel elements in diffuse-porous species mature more than a month after leaf expansion (Suzuki et al. 1996), or around the time of full leaf expansion (Suzuki et al. 2000). Thus, the timing of vessel maturation clearly differs between ring-porous and diffuse-porous species.
Many previous studies of wood anatomy have not considered the stages of leaf development or patterns of leaf appearance. Similarly, many ecological studies have not investigated the process of vessel formation or vessel microstructure. To clarify the relationship between leaf phenology and vessel formation from the ecophysiological viewpoint, both traits need to be examined concurrently to determine how they relate to photosynthesis and water transport. Such studies in natural forests are very limited, as are studies of seasonal changes in vessel formation in individual trees over a year in both the twigs and stems of ring- and diffuse-porous species. Suzuki et al. (1996, 2000) examined seasonal vessel formation in stems only, and Ladefoged (1952) examined cell division, but not lignification, in different trees over the course of a year.
To examine the anatomical relationship between leaf phenology and vessel formation from an ecophysiological viewpoint, relating photosynthesis and water transport, we examined vessel lignification at the time of leaf appearance and full leaf expansion. This study examines the phenology of the entire tree, unlike the shoot-level phenology of earlier studies (e.g., Marks 1975; Bicknell 1982; Kikuzawa 1983). We used an increment borer to sample stems and to observe the phenology of vessel lignification of trees.
This is a case study of the timing of budburst in relation to xylem vessel development in both twigs and stem wood for species of ring- and diffuse-porous deciduous trees growing together in a temperate forest in central Japan. The objective of this study is to clarify how different aspects of plant function are coordinated developmentally.
Materials and methods
Study site and sample trees
The study was conducted at the Kyoto University Forest in Ashiu, Kyoto Prefecture, central Japan at 35°18′N, 135°43′E. This is a cool temperate forest, and the dominant species are Fagus crenata and Quercus mongolica var. grosseserrata. The mean annual temperature over 30 years (1971–2000) at the meteorological station (356-m elevation) was 11.7 °C with mean minimum and maximum temperatures of −0.2 and 24.2 °C, respectively. Mean annual precipitation was 2,353 mm, most of which occurred as winter snow (Forest Research Station of Graduate School of Agriculture, Kyoto University 2002). The study site is at 650–670 m elevation, with mean temperatures about 2 °C cooler than at the meteorological station in 2004. The minimum temperatures were −12.3 °C in 2004 and −14.7 °C in 2005 (Tyozidani Meteorological Reference Materials for Business Use, Unpublished data). The study area comprised 20 m on either side of a 1-km portion of a path in a flat-bottomed, south-facing valley. All sample trees were thus exposed to similar weather conditions.
Sample trees (Table 1) were selected from canopy trees of four ring-porous species, Castanea crenata, Fraxinus mandshurica, Quercus mongolica var. grosseserrata, and Zelkova serrata, and five diffuse-porous species, Aesculus turbinata, Betula grossa, Cercidiphyllum japonicum, Fagus crenata, and Pterocarya rhoifolia. In 2005, we added another ring-porous species, Hovenia tomentella. Scientific names are based on those reported by Hayashi et al. (1987) except for H. tomentella (Meyer and Walker 1984). Porosity classes were defined from Hayashi (1991), except for H. tomentella, which was determined by visual examination of pores. Study trees were selected from individuals with relatively straight stems and many leaves, 18–74 cm in stem diameter at breast height and 9–31 m tall. All species occur naturally in Ashiu, except for F. mandshurica, which is non-native and was planted. Z. serrata trees were planted individuals.
Sampling
Cylindrical wood core samples (7 mm in diameter, 20 mm in length) were collected at breast height (1.3 ± 0.2 m above ground) with an increment borer (Mattson) from five trees of each species in 2004 and 2005 (except that in 2005, four trees were sampled for Q. mongolica var. grosseserrata and six trees for C. japonicum).
Twigs with sun leaves were collected from each tree using 3-m-long pruners and a ladder in 2004, or 12-m-long pruners in 2005. One tree of each species was sampled in 2004 and three or more trees of each species were sampled in 2005 (except that only one tree of H. tomentella was sampled in 2005). In this study, twigs that were newly extended within the previous year were regarded as 1-year-old twigs. We observed vessel formation in 1-year-old twigs in 2004 and in 1-year-old (or occasionally 2- or 3-year-old) twigs in 2005 at 0.5–1.5 cm from bud bases. Stem cores and twigs were fixed with 3 % aqueous glutaraldehyde soon after sampling.
In 2004, samples were collected biweekly between April 22 and July 1 and monthly between July 22 and November 16, for a total of 11 samplings. In 2005, samples were collected weekly between April 19 and 28 and biweekly between April 28 and July 21, for a total of eight samplings.
Assessment of vessel formation
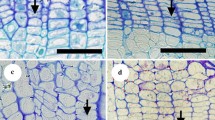
Transverse sections of 15–30 μm thickness were cut using a sliding microtome. They were then double-stained with 1 % safranine and 1 % fast green, in preparation for light microscopy (Sass 1951).
Vessel development begins with cambial cell division, followed by enlargement of vessel elements, secondary cell wall deposition, secondary cell wall lignification, and then disintegration of the end walls (Fukushima et al. 2003). In this study, lignification of the first-formed vessels—those formed first in the current growth ring, often adjacent to the annual ring border—was determined by the presence of red staining with safranine (Takahashi et al. 2008). When double staining was ambiguous for lignification, a phloroglucinol–hydrochloric acid reaction was used (Takahashi et al. 2008). The date of lignification was recorded as the first date on which lignification of almost all of the first-formed vessels was observed.
Observation of leaf phenology
The leaves of each sample tree were observed through binoculars (Nikon 8 × 30, 8.8°WF) and photographed in situ on the tree and on the sampled twigs. The observed phenology parameters were as follows. Leaf appearance for a leaf was defined as when the lamina separated from the shoot axis (Kikuzawa 1983), and full leaf expansion for a leaf as when the leaf area ceased to increase based on the definition of Suzuki et al. (2000). We also distinguished flush-leaves and successive-leaves in a tree (Kikuzawa 1983; Miyazawa and Kikuzawa 2004). The former are leaves that appear almost simultaneously in a short period and flush immediately after budbreak in early spring (i.e., early leaves). The latter are leaves that appear one by one successively over a long period (i.e., late leaves). The type of leaf emergence pattern of each species was determined by seasonal observation of sample trees, and was based on the types reported by Kikuzawa (1983): flush type, succeeding type, and intermediate type. In this study, flush and intermediate types were observed (Table 1). Species with a flush-type emergence pattern had only flush-leaves, and species with an intermediate-type emergence pattern had both flush-leaves and successive-leaves (Table 1). Leaf appearance and full leaf expansion at a whole-tree level were observed in this study, and these terms are used at the level of the whole tree throughout the rest of this paper unless otherwise indicated. The dates of leaf appearance and full leaf expansion for an individual tree were recorded as the first observation date on which almost all of the flush-leaves on the tree had appeared and had fully expanded, respectively. Here, leaf appearance clearly differed from full leaf expansion in terms of the density of greenness.
Results
Timing of vessel lignification in twigs relative to leaf appearance
Lignification of the first-formed vessels in twigs was observed 0–2 weeks after leaf appearance in both ring- and diffuse-porous species in 2004, and 0–2 weeks before leaf appearance in ring-porous species and 2 weeks before to 2 weeks after leaf appearance in diffuse-porous species in 2005 (Fig. 1). The timing of vessel lignification was not significantly different between ring- and diffuse-porous species in 2004, but was significantly different in 2005 (p < 0.01, Table 2). Four of the 20 ring-porous trees sampled showed lignification of vessels prior to leaf appearance, although one Q. mongolica individual did not show lignification until 2 weeks after leaf appearance. On the other hand, of the 24 diffuse-porous trees, 11 showed lignification after leaf appearance, although two samples (one A. turbinata and one F. crenata) showed lignification prior to leaf appearance. The timing in 2005 was somewhat earlier than that in 2004.
The timing of lignification of the first-formed vessels in twigs was around the time of leaf appearance (±2 weeks) in both ring- and diffuse-porous species. Lignification of the first-formed vessels in twigs relative to leaf appearance tended to occur 1.1 weeks earlier in ring-porous species than in diffuse-porous species (Table 2).
Timing of vessel lignification in stems relative to leaf appearance
Lignification of the first-formed vessels in stems was observed 2 weeks before to 4 weeks after leaf appearance in ring-porous species, and 2–8 weeks (2004) or 4–8 weeks (2005) after leaf appearance in diffuse-porous species (Fig. 2). Ring-porous species showed significantly earlier vessel lignification than diffuse-porous species in both 2004 and 2005 (p < 0.01, Table 2). Of the ring-porous species, three individuals (two Q. mongolica in 2004 and one H. tomentella in 2005) showed lignification 4 weeks after leaf appearance. Across all ring-porous species, 16 of 44 samples examined showed lignification after leaf appearance. Nine of ten F. mandshurica samples and one C. crenata (2004) had lignification 2 weeks before leaf appearance. In contrast, among the diffuse-porous species, two B. grossa (2004) had lignification 2 weeks after leaf appearance, and no new vessels were observed in one C. japonicum and one F. crenata in 2004 and one C. japonicum in 2005 (Table 1) (Takahashi et al. 2008, describe some possible reasons for the latter occurrence).
The time of the lignification of the first-formed vessels in stems relative to the time of leaf appearance was 5.4 weeks (2004) or 5.3 weeks (2005) earlier in ring-porous species than in diffuse-porous species (Table 2).
Timing of vessel lignification in stems and twigs
Compared to the timing of lignification of vessels in twigs, lignification in stems occurred 0–2 weeks earlier in 2004 or within 2 weeks in 2005 in ring-porous species and 2–6 weeks (2004) or 2–8 weeks (2005) after in diffuse-porous species (Fig. 3). Lignification was 5.3 weeks (2004) or 4.3 weeks (2005) earlier in ring-porous species (p < 0.01, Table 2). Three of six F. mandshurica samples had lignification in stems 2 weeks before in twigs. Lignification of vessels in stems was later than that in twigs in six of 20 ring-porous trees, a pattern similar to that of all diffuse-porous trees.
Timing of vessel lignification in twigs and stems relative to full leaf expansion
The first-formed vessels in twigs were lignified 2–4 weeks before full leaf expansion in ring-porous species and 0–2 weeks (2004) or 0–4 weeks (2005) before full leaf expansion in diffuse-porous species (Fig. 4). Differences in the timing of vessel lignification between ring- and diffuse-porous species were significant in 2005 (0.01 < p < 0.05) but not in 2004 (Table 2). Ring-porous species showed vessel lignification 1.1 weeks earlier than diffuse-porous species in 2005 (Table 2). Several diffuse-porous trees had lignification concurrent with full leaf expansion.
Lignification of vessels in stems occurred 4 weeks before to 0 weeks after (2004) or 4 weeks before to 2 weeks after (2005) full leaf expansion in ring-porous species, 5.5 weeks (2004) or 5.3 weeks (2005) earlier than in diffuse-porous species (p < 0.01), which showed lignification of vessels 0–6 weeks (2004) or 0–8 weeks (2005) after full leaf expansion (Fig. 5; Table 2). Across all of the ring-porous species, one H. tomentella showed lignification 2 weeks after full leaf expansion.
Discussion
Summary of timing of leaf and vessel formation
Figure 6 summarizes the timing of vessel formation in twigs and stems in relation to leaf phenology. The first-formed vessels in twigs were lignified around the time of leaf appearance at the whole-tree level in the case of flush-leaves and at or before full leaf expansion of the tree in both ring- and diffuse-porous species, although the timing tended to be slightly later in diffuse-porous species (Figs. 1, 4). In almost all ring-porous species, stem vessels were lignified starting within a short time of leaf appearance and ending by the time of full leaf expansion (Figs. 2, 5). F. mandshurica had the earliest lignification, with individuals typically showing lignification prior to leaf appearance, and H. tomentella had somewhat later lignification. In contrast, lignification of stem vessels in diffuse-porous species occurred long after leaf appearance and full leaf expansion (Figs. 2, 5). Overall, lignification of vessels in stems occurred concurrently or soon after lignification of vessels in twigs in ring-porous species but relatively later in diffuse-porous species (Fig. 3). F. mandshurica had somewhat earlier lignification in stems in relation to twigs.
Order and time-lag of leaf and vessel formation
It has generally been reported that the timing of vessel formation in relation to leaf phenology differs between ring-porous and diffuse-porous species. Studies on ring-porous species have shown completion of secondary wall deposition in stems between 1 week before and 3 weeks after leaf expansion (Suzuki et al. 1996), or maturation between 1 week before and 1 week after leaf budding (Suzuki et al. 2000), coinciding with the unfolding of the first leaves (Priestley et al. 1933; Zasada and Zahner 1969). In diffuse-porous species, secondary wall deposition occurred 4–9 weeks after leaf expansion (Suzuki et al. 1996). In this study, we found that lignification of the first-formed vessels in stems occurred between 2 weeks before and 4 weeks after leaf appearance in the case of flush-leaves in ring-porous species, and 2–8 weeks after leaf appearance in diffuse-porous species (Fig. 2). Thus, this study confirms previous findings that vessel formation tends to occur earlier in ring-porous species than in diffuse-porous species.
Vessel elements in twigs begin to lignify before buds open (Zasada and Zahner 1969), and cell division at bud bases begins before or simultaneously with budbreak in both ring- and diffuse-porous species (Ladefoged 1952), indicating no difference in timing between ring- and diffuse-porous species. However, in this study, ring-porous species showed significantly earlier vessel formation in twigs than diffuse-porous species in 2005 (Table 2). Lignification of the first-formed vessels in twigs occurred somewhat earlier in ring-porous species than in diffuse-porous species relative to the timing of leaf appearance in species with flush-leaves (Fig. 1). In relation to the timing of full leaf expansion, this study indicated that lignification of vessels in twigs occurred before full leaf expansion in ring-porous species and before or concurrent with full leaf expansion in diffuse-porous species (Fig. 4). Thus, in 1-year-old twigs 0.5–1.5 cm from bud bases, the same as in stems, there was a difference in the timing of vessel lignification in relation to the timing of leaf phenology between ring-porous and diffuse-porous species.
Ladefoged (1952) showed that cell division occurs more rapidly in the basipetal direction in ring-porous species than in diffuse-porous species, and so there is a relatively long period between the onset of cell division at the bud and stem bases in diffuse-porous species. In this study, we also found that the lignification of vessels occurred later in stems than in twigs in both ring-porous and diffuse-porous species, but the time-lag was longer in diffuse-porous species (Fig. 3). Other studies have shown that the lignification of vessels in twigs and stems lower in the tree occurs prior to bud opening in ring-porous species (Zasada and Zahner 1969). In this study, the first-formed vessels in twigs were lignified by the time of leaf appearance in nearly all of the ring-porous trees examined (Fig. 1), while only some trees showed lignification in stems at this time (Fig. 2). This suggests that not all of the first-formed vessels are needed for water transport in stems at the time of leaf appearance in ring-porous species with flush-leaves, though all of them are needed in twigs.
The development stages of ring-porous species can be summarized as follows: lignification of the first-formed vessels in twigs occurs first, followed by the appearance of flush-leaves, lignification of stem vessels, and full expansion of leaves (occasionally lignification of stem vessels occurs before leaf appearance). The development stages of diffuse-porous species can be summarized as follows: the appearance of leaves occurs first, followed by lignification of twig vessels, full expansion of leaves, and lignification of stem vessels (Fig. 6). The time-lag between the lignification of the first-formed vessels in twigs and leaf appearance is short or absent in both ring- and diffuse-porous species, but the time-lag between the lignification of stem vessels and nearly simultaneous occurrences of leaf appearance and lignification of vessels in twigs is short or absent in ring-porous species and long in diffuse-porous species. The time-lag between lignification in twigs or stems and full leaf expansion is variable in both ring- and diffuse-porous species.
Differences in physiological traits in ring- and diffuse-porous species
In early spring, transpiration begins in leaves as soon as they open, and transpiration and photosynthetic capacities increase rapidly as leaves expand and their leaf area increases (Šesták 1985; Koike 1990; Miyazawa and Terashima 2001; Muraoka and Koizumi 2005). This creates a temporal relationship between leaf phenology and vessel formation.
Almost all ring-porous species tend to lignify the first-formed vessels in twigs before or simultaneous with the appearance of flush-leaves and before full expansion of the leaves (Figs. 1, 4). This suggests that the first-formed vessels in twigs are ready for water transport by the time transpiration is needed by flush-leaves. Further, in the case of the tree that did not show lignification of vessels until 2 weeks after leaf appearance, it was still prepared for water transport before leaves reached maximal transpiration.
In contrast to vessels in twigs, vessels in the stems of ring-porous species were lignified 0–4 weeks after (or somewhat before) the appearance of flush-leaves and before or concurrently with full expansion of these leaves (Figs. 2, 5). This suggests that the first-formed vessels in stems are prepared to transport water while the transpiration and photosynthetic capacities of the leaves are rapidly increasing during leaf expansion. It is vital that trees complete the first-formed vessels in twigs and stems before near maximal transpiration is reached at the time of full leaf expansion, because wide vessels transport water only in the year they develop (Greenidge 1955; Chaney and Kozlowski 1977; Ellmore and Ewers 1986; Utsumi et al. 1999; Umebayashi et al. 2008). At temperatures that occur at the present study site, the wide vessels of ring-porous species may become embolized by the freezing of xylem (Zimmermann 1964; Sperry et al. 1994; Utsumi et al. 1996). Thus, all of the first-formed vessels in twigs and stems prepare to transport water by the time when the flush-leaves reach near-maximal rates of transpiration, and allow photosynthesis to proceed smoothly. In H. tomentella, the first stem vessels are not prepared for transporting water until 0–2 weeks after the leaves have fully expanded. This may be related to the phenology of successive-leaves produced after flush-leaves open (unpublished data).
In diffuse-porous species, lignification of twig vessels occurred within 2 weeks of leaf appearance and at or before full leaf expansion (Figs. 1, 4). This suggests that the first-formed vessels in twigs prepare to transport water while transpiration and photosynthesis are rapidly increasing, and are functional by the time the near-maximum capacity of the leaves is reached. This is similar to the stems of ring-porous species.
Diffuse-porous species did not show lignification of the first stem vessels until 2–8 weeks after the appearance of flush-leaves (Fig. 2). Other studies have shown that the lignification of stem vessels occurred around the time of full leaf expansion in diffuse-porous species (Suzuki et al. 2000). However, the present study found that lignification of stem vessels did not occur until several weeks (up to 8) after full expansion of these leaves (Fig. 5). This suggests that the first stem vessels in these species prepare to transport water after the transpiration and photosynthetic capacities of these leaves have increased to near-maximal levels. Diffuse-porous species can do this because vessels in several outer rings, not just the current year’s ring, can transport water (Greenidge 1955; Chaney and Kozlowski 1977; Utsumi et al. 1998; Umebayashi et al. 2008). Thus, the first-formed vessels in twigs are prepared to transport water by the time transpiration needs are near-maximum. Stem vessels are prepared after the rapid increase in transpiration and allow photosynthesis to proceed at a steady rate.
In summary, the ring-porous species produce single-year leaves and most functional vessels intensively in a short period; whereas the diffuse-porous species produce single-year leaves and multiple-year vessels less intensively.
This study clarified the relationship between the timing of vessel formation and leaf phenology in early spring in ring-porous and diffuse-porous species. Diffuse-porous and ring-porous species generally followed similar patterns of vessel-leaf phenology within each group. The diffuse-porous species P. rhoifolia has wider early vessels than other diffuse-porous species, but still followed the same patterns as other diffuse-porous species. On the other hand, there were some fluctuations by species or by year within ring- or diffuse-porous species. It is unclear why some individuals of F. mandshurica or H. tomentella formed vessels in stems earlier or later than other ring-porous trees. Similarly, the timing of vessel formation in twigs fluctuated between the years. It is also unclear how these patterns in the timing of vessel formation compare to those in trees with other types of wood porosity, such as semi-ring-porous species. More studies are needed to clarify the relationships between the timing of vessel formation and leaf phenology of species with different wood porosities in different climatic regions.
References
Bicknell SH (1982) Development of canopy stratification during early succession in northern hardwoods. For Ecol Manage 4:41–51
Chaney WR, Kozlowski TT (1977) Patterns of water movement in intact and excised stems of Fraxinus americana and Acer saccharum seedlings. Ann Bot 41:1093–1100
Ellmore GS, Ewers FW (1986) Fluid flow in the outermost xylem increment of a ring-porous tree, Ulmus americana. Am J Bot 73:1771–1774
Fahn A, Werker E (1990) Seasonal cambial activity. In: Iqbal M (ed) The vascular cambium. Research Studies Press, Taunton, pp 139–157
Forest Research Station of Graduate School of Agriculture, Kyoto University (2002) Meteorological observations in the Kyoto University forests No. 13, 1996–2000
Frankenstein C, Eckstein D, Schmitt U (2005) The onset of cambium activity—a matter of agreement? Dendrochronologia 23:57–62
Friesner RC (1942) Dendrometer studies on five species of broadleaf trees in Indiana. Butler Univ Bot Stud 5:160–172
Fukushima K, Funada R, Sugiyama J, Takabe K, Umezawa T, Yamamoto H (2003) Secondary xylem formation—introduction to biomass science. Kaiseisha Press, Otsu, pp 33–39 (in Japanese)
Greenidge KNH (1955) Studies in the physiology of forest trees. III. The effect of drastic interruption of conducting tissues on moisture movement. Am J Bot 42:582–587
Hayashi S (1991) Micrographic atlas of Japanese woods. Wood Research Institute, Kyoto University 23:1–147 (in Japanese)
Hayashi Y, Furusato K, Nakamura T (1987) Illustrated trees in colour. Hokuryukan, Japan (in Japanese)
Imagawa H, Ishida S (1972) Study on the wood formation in trees: report III. Occurrence of the overwintering cells in cambial zone in several ring-porous trees. Res Bull Coll Exp For Hokkaido Univ 29:207–221 (in Japanese with English summary)
Kikuzawa K (1983) Leaf survival of woody plants in deciduous broad-leaved forests. 1. Tall trees. Can J Bot 61:2133–2139
Koike T (1990) Autumn coloring, photosynthetic performance and leaf development of deciduous broad-leaved trees in relation to forest succession. Tree Physiol 7:21–32
Komiyama A (1991) Relationships between stem-diameter growth periods and leaf growth periods of deciduous broadleaved tree species with reference to environmental factors. J Jpn For Soc 73:409–418 (in Japanese with English abstract)
Komiyama A, Inoue S, Ishikawa T (1987) Characteristics of the seasonal diameter growth of twenty-five species of deciduous broad-leaved trees. J Jpn For Soc 69:379–385 (in Japanese with English abstract)
Ladefoged K (1952) The periodicity of wood formation. Dan Biol Skr 7:1–98
Marks PL (1975) On the relation between extension growth and successional status of deciduous trees of the northeastern United States. Bull Torrey Bot Club 102:172–177
Maruyama K, Oida S, Fukumoto Y, Kamitani T (1992) Annual life histories of various tall deciduous tree species viewed from vegetative growth. Bull Niigata Univ For 25:35–68 (in Japanese with English summary)
Meyer FG, Walker EH (1984) Flora of Japan (in English). Jisaburo Ohwi National Science Museum/Japan Smithsonian Institution, Tokyo/Washington, DC
Miyazawa Y, Kikuzawa K (2004) Phenology and photosynthetic traits of short shoots and long shoots in Betula grossa. Tree Physiol 24:631–637
Miyazawa S-I, Terashima I (2001) Slow development of leaf photosynthesis in an evergreen broad-leaved tree, Castanopsis sieboldii: relationships between leaf anatomical characteristics and photosynthetic rate. Plant Cell Environ 24:279–291
Muraoka H, Koizumi H (2005) Photosynthetic and structural characteristics of canopy and shrub trees in a cool-temperate deciduous broadleaved forest: implication to the ecosystem carbon gain. Agric For Meteorol 134:39–59
Priestley JH, Scott LI (1936) A note upon summer wood production in the tree. Proc Leeds Philos Lit Soc 3:235–248
Priestley JH, Scott LI, Malins ME (1933) A new method of studying cambial activity. Proc Leeds Philos Lit Soc 2:365–374
Sass JE (1951) Botanical microtechnique, 2nd edn. The Iowa State College Press, USA, pp 69–71
Šesták Z (1985) Photosynthesis during leaf development. Dr W Junk Publishers, The Hague
Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE (1994) Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of northern Utah and interior Alaska. Ecology 75:1736–1752
Suzuki M, Yoda K, Suzuki H (1996) Phenological comparison of the onset of vessel formation between ring-porous and diffuse-porous deciduous trees in a Japanese temperate forest. IAWA 17:431–444
Suzuki M, Hirano R, Yoda K (2000) Phenological analysis of wood formation in temperate deciduous ring and diffuse porous wood. Chonnam National University Press, Kwangju (printed in Korea, Reprint from Kim YS (ed) New Horizons in Wood Anatomy, pp 132–137)
Takahashi S, Okada N, Nobuchi T (2008) Examination of wood sampling method with an increment borer: an investigation of seasonal changes in vessel formation. For Res Kyoto 77:123–128
Tepper HB, Hollis CA (1967) Mitotic reactivation of the terminal bud and cambium of white ash. Science 156:1635–1636
Umebayashi T, Utsumi Y, Koga S, Inoue S, Fujikawa S, Arakawa K, Matsumura J, Oda K (2008) Conducting pathways in north temperate deciduous broadleaved trees. IAWA 29:247–263
Utsumi Y, Sano Y, Ohtani J, Fujikawa S (1996) Seasonal changes in the distribution of water in the outer growth rings of Fraxinus mandshurica var. japonica: a study by cryo-scanning electron microscopy. IAWA 17:113–124
Utsumi Y, Sano Y, Fujikawa S, Funada R, Ohtani J (1998) Visualization of cavitated vessels in winter and refilled vessels in spring in diffuse-porous trees by cryo-scanning electron microscopy. Plant Physiol 117:1463–1471
Utsumi Y, Sano Y, Funada R, Fujikawa S, Ohtani J (1999) The progression of cavitation in earlywood vessels of Fraxinus mandshurica var japonica during freezing and thawing. Plant Physiol 121:897–904
Wareing PF (1950) Extension and radial growth in trees. Nature 166:278
Wheeler EA, Baas P, Gasson PE (1989) IAWA list of microscopic features for hardwood identification with an appendix on non-anatomical information. IAWA Bull 10:219–332
Wilcox H (1962) Cambial growth characteristics. In: Kozlowski TT (ed) Tree growth. The Ronald Press Company, New York, pp 57–88
Zasada JC, Zahner R (1969) Vessel element development in the earlywood of red oak (Quercus rubra). Can J Bot 47:1965–1971
Zimmermann MH (1964) Sap movements in trees. Biorheology 2:15–27
Acknowledgments
We thank the staff of the Ashiu Forest Research Station of the Field Science Education and Research Center, Kyoto University, for access to sample trees. Professor K. Takabe and Assistant Professor A. Yoshinaga of the Laboratory of Tree Cell Biology at the Graduate School of Agriculture, Kyoto University, helped with the phloroglucinol–hydrochloric acid reaction and this laboratory allowed use of a freezing microtome. Professor Y. Okumoto of the Laboratory of Plant Breeding, Graduate School of Agriculture, Kyoto University, provided advice on statistical analyses. Mr. T. Niro assisted with sample collection and other students of the Laboratory of Forest Utilization, Graduate School of Agriculture, Kyoto University, provided assistance at various stages of the study. We also thank Professor A. Osawa of this laboratory and Assistant Professor E. Takahashi of the Laboratory of Faculty of Life and Environmental Science, Shimane University, for critical reading of the manuscript and helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Takahashi, S., Okada, N. & Nobuchi, T. Relationship between the timing of vessel formation and leaf phenology in ten ring-porous and diffuse-porous deciduous tree species. Ecol Res 28, 615–624 (2013). https://doi.org/10.1007/s11284-013-1053-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-013-1053-x