Abstract
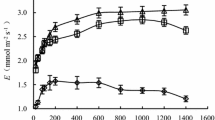
In the Mediterranean basin, Tamarix spp. constitute important populations along rivers and sea coasts, and might be primarily subjected to water level fluctuations and salinization, as a consequence of global climate change. Here, we analyze leaf gas exchange and xylem anatomy during a water level decrease below the soil surface after short-term flooding with fresh- and saline-water (200 mM) in order to predict Tamarix africana Poiret responses under future environmental conditions. Fresh-water level reduction negatively affected stomatal conductance (−56.3 %), but only when water decreased to the lowest level (15 cm below the soil surface). No effects on assimilation rates and xylem vessel dimensions occurred. Under saline conditions, the rate of the water level decrease was lower compared to the non-saline treatment, as stomatal conductance was negatively affected by salinity (−59.5 %) and significantly declined over time. Moreover, decreases in mean xylem vessel area (−51.3 %), assimilation rates (−52.2 %) and stomatal conductance (−76.0 %) were also observed compared to the control, indicating both an osmotic stress and a toxic effect of NaCl on leaf gas exchange. These leaf responses were probably induced by greater belowground-root salt absorption and transport compared to previous flooding conditions, as confirmed by the increase in salt excretion (+473.2 %). The results emphasize the survival risk of Tamarix spp. to water level variation under both saline and non-saline conditions, and the need of management practices focused on the conservation of these populations.






Similar content being viewed by others
References
Abou Jaoudé R, de Dato G, Palmegiani M, De Angelis P (2012) Impact of fresh and saline water flooding on leaf gas exchange in two Italian provenances of Tamarix africana Poiret. Plant Biol. doi:10.1111/j.1438-8677.2012.00597.x
Ainsworth EA, Davey PA, Hymus GJ, Drake BG, Long SP (2002) Long-term response of photosynthesis to elevated carbon dioxide in a Florida scrub-oak ecosystem. Ecol Appl 12:1267–1275
Akilan K, Marshall JK, Morgan AL, Farrell RCC, Bell DT (1997) Restoration of catchment water balance: responses of clonal river red gum (Eucalyptus camaldulensis) to waterlogging. Restor Ecol 5:101–108
Arizpe D, Mendes A, Rabaça JE (2008) Sustainable riparian zones. A management guide. Generalitat Valenciana, Spain
Bar-Nun N, Poljakoff-Mayber A (1974) Some aspects of protein metabolism in Tamarix tetragyna roots grown in a saline environment. Aust J Plant Physiol 1:237–246
Barrett-Lennard EG (2003) The interaction between waterlogging and salinity in higher plants: causes, consequences and implications. Plant Soil 253:35–54
Blom CWPM, Voesenek LACJ (1996) Flooding: the survival strategies of plants. Trends Ecol Evol 11:290–295
Bongi G, Loreto F (1989) Gas-exchange properties of salt-stressed olive (Olea europaea L.) leaves. Plant Physiol 90:1408–1416
Boursier P, Läuchli A (1990) Growth responses and mineral nutrient relations of salt stressed sorghum. Crop Sci 30:1226–1233
Braendle R, Crawford RMM (1999) Plants as amphibians. Perspect Plant Ecol Evol Syst 2:56–78
Brotherson JD, Field D (1987) Tamarix: impacts of a successful weed. Rangelands 9:110–112
Centritto M, Loreto F, Chartzoulakis K (2003) The use of low [CO2] to estimate olive saplings. Plant Cell Environ 26:585–594
Chen HJ, Qualls RG, Blank RR (2005) Effect of soil flooding on photosynthesis, carbohydrate partitioning and nutrient uptake in the invasive exotic Lepidium latifolium. Aquat Bot 82:250–268
Cleverly JR, Smith SD, Sala A, Devitt DA (1997) Invasive capacity of Tamarix ramosissima in a Majove Desert floodplain. The role of drought. Oecologia 111:12–18
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36
Day JW, Christian RR, Boesch DM, Yáñez-Arancibia A, Morris J, Twilley RR, Naylor L, Schnaffner L, Stevenson C (2008) Consequences of climate change on the ecogeomorphology of coastal wetlands. Estuar Coast 31:477–491
DeLaune RD, Jugsujinda A, Peterson G, Patrick W (2003) Impact of Mississippi River freshwater reintroduction on Spartina patens marshes: responses to nutrient input and lowering of salinity. Wetlands 25:155–161
Di Tomaso JM (1998) Impact, biology, and ecology of saltcedar (Tamarix spp.) in the Southwestern United States. Weed Tech 12:326–336
Drew MC, He CJ, Morgan PW (2000) Programmed cell death and aerenchyma formation in roots. Trends Plant Sci 5:123–127
Evans DE (2004) Aerenchyma formation. New Phytol 161:35–49
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Fernandez MD (2006) Changes in photosynthesis and fluorescence in response to flooding in emerged and submerged leaves of Pouteria orinocoensis. Photosynthetica 44:32–38
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963
Ginzburg C (1967) Organization of the adventitious root apex in Tamarix aphylla. Am J Bot 54:4–8
Glenn E, Tanner R, Mendez S, Kehret T, Moore D, Garcia J, Valdes C (1998) Growth rates, salt tolerance characteristics of native and invasive riparian plants from the delta of Colorado River, Mexico. J Arid Environ 40:271–294
Gries D, Zeng F, Arndt SK, Bruelheide H, Thomas FM, Zhang X, Runge M (2003) Growth and water relations of Tamarix ramosissima and Populus euphratica on Taklamakan desert dunes in relation to depth to a permanent water table. Plant Cell Environ 26:725–736
Gries D, Foetzki A, Arndt SK, Bruelheide H, Thomas FM, Zhang X, Runge M (2005) Production of perennial vegetation in an oasis-desert transition zone NW China—allometric estimation, and assessment of flooding and use effects. Plant Ecol 181:23–43
Hacke UG, Sperry JS, Wheeler JK, Castro L (2006) Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol 26:689–701
Hirano T, Kiyota M, Aiga I (1995) Physical effects of dust on leaf physiology of cucumber and kidney bean plants. Environ Pollut 89:255–261
Horton JA (1960) The ecology of saltcedar. Proc Arizona Watershed Symp 4:19–21
Horton JL, Kolb TE, Hart SC (2001) Leaf gas exchange characteristics differ among Sonoran Desert riparian tree species. Tree Physiol 21:233–241
IPCC (2007) Climate change 2007: the physical science basis. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Junghans U, Polle A, Düchting P, Weiler E, Kuhlman B, Gruber F, Teichmann T (2006) Adaptation to high salinity in poplar involves changes in xylem anatomy and auxin physiology. Plant Cell Environ 29:1519–1531
Kleinkopf GE, Wallace A (1974) Physiological basis for salt tolerance in Tamarix ramosissima. Plant Sci Lett 3:157–163
Kozlowski TT (1997) Responses of woody plants to flooding and salinity. Tree Physiol Monogr 1:1–29
Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54:2393–2401
Loreto F, Centritto M, Chartzoulakis K (2003) Photosynthetic limitations in olive cultivars with different sensitivity to salt stress. Plant Cell Environ 26:595–601
Lovelock CE, Ball MC (2002) Influence of salinity on photosynthesis of halophytes. In: Läuchli A, Lüttge U (eds) Salinity: environment, plants, molecules. Kluwer Academic Publishers, The Netherlands, pp 315–339
Mansour MMF (2000) Nitrogen containing compounds and adaptation of plants to salinity stress. Biol Plant 43:491–500
Marcar NE, Crawford DF, Saunders A, Matheson AC, Arnold RA (2002) Genetic variation among and within provenances and families of Eucalyptus grandis W. Hill and E. Globosus Labill. subsp globosus seedlings in response to salinity and waterlogging. Forest Ecol Manag 162:231–249
Mc Leod KW, McCarron JK, Conner WH (1999) Photosynthesis and water relations of four oak species: impact of flooding and salinity. Trees Struct Funct 13:178–187
Merritt DM, LeRoy Poff N (2010) Shifting dominance of riparian Populus and Tamarix along gradients of flow alteration in western North American rivers. Ecol Appl 20:135–152
Meyer RF, Boyer JS (1972) Sensitivity of cell division and cell elongation to low water potentials in soybean hypocotyls. Planta 108:77–87
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Nandy Datta P, Das S, Ghose M, Spooner-Hart R (2007) Effects of salinity on photosynthesis, leaf anatomy, ion accumulation and photosynthetic nitrogen use efficiency in five Indian mangroves. Wetl Ecol Manag 15:347–357
Parida AK, Das AB, Mittra B (2004) Effects of salt on growth, ion accumulation, photosynthesis and leaf anatomy of the mangrove, Bruguiera parviflora. Trees Struct Funct 18:167–174
Pezeshki SR, Pardue JH, DeLaune RD (1996) Leaf gas exchange and growth of flood-tolerant and flood-sensitive tree species under low soil redox conditions. Tree Physiol 16:453–458
Rengifo E, Tezara W, Herrera A (2005) Water relations, chlorophyll a fluorescence, and contents of saccharides in tree species of a tropical forest in response to flood. Photosynthetica 43:203–210
Salter J, Morris K, Boon PI (2008) Does salinity reduce the tolerance of two contrasting wetland plants, the submerged monocot, Vallisneria australis and the woody shrub Melaleuca ericifolia, to wetting and drying? Mar Freshw Res 59:291–303
Salter J, Morris K, Read J, Boon PI (2010) Impact of long-term, saline flooding on condition and reproduction of the clonal wetland tree, Melaleuca ericifolia (Myrtaceae). Plant Ecol 206:41–57
Saqib M, Akhtar J, Qureshi RH (2005) Na+ exclusion and salt resistance of wheat (Triticum aestivum) in saline-waterlogged conditions are improved by the development of adventitious nodal roots and cortical root aerenchyma. Plant Sci 169:125–130
Schmitz N, Jansen S, Verheyden A, Kairo JG, Beeckman H, Koedam N (2007) Comparative anatomy of the intervessel pits in two mangrove species growing along a natural salinity gradient in Gazi Bay, Kenya. Ann Bot 100:271–281
Schweingruber FH, Börner A, Schulze ED (2008) Atlas of woody plant stems: evolution, structure, and environmental modifications. Springer, Heidelberg
Sobrado MA (2007) Relationship of water transport to anatomical features in the mangrove Laguncularia racemosa grown under contrasting salinities. New Phytol 173:584–591
Sprenger MD, Smith LM, Taylor JP (2001) Testing control of saltcedar seedlings using fall flooding. Wetlands 21:437–441
Stromberg JC, Lite SJ, Marler R, Paradzich C, Shafroth PB, Shorrock D, White JM, White MS (2007) Altered stream-flow regimes and invasive plant species: the Tamarix case. Global Ecol Biogeogr 16:381–393
Tallent-Halsell NG, Walker LR (2002) Responses of Salix gooddingii and Tamarix ramosissima to flooding. Wetlands 22:776–785
Ueda A, Kanechi M, Uno Y, Inagaki N (2003) Photosynthetic limitations of a halophyte sea aster (Aster tripolium L.) under water stress and NaCl stress. J Plant Res 116:65–70
Vandersande MW, Glenn EP, Walworth JL (2001) Tolerance of five riparian plants from the lower Colorado River to salinity drought and inundation. J Arid Environ 49:147–159
Visser EJW, Heijink CJ, Vanhout KJGM, Voesenek LACJ, Barendse GWM, Blom CWPM (1995) Regulatory role of auxin in adventitious root-formation in 2 species of Rumex, differing in their sensitivity to waterlogging. Physiol Plant 93:116–122
Waisel Y (1961) Ecological studies on Tamarix aphylla (L.) Karst. III. The salt economy. Plant Soil 13:356–363
Wang CH, Lu M, Yang B, Yang Q, Zhang XD, Hara T, Li B (2010) Effects of environmental gradients on the performances of four dominant plants in a Chinese saltmarsh: implications for plant zonation. Ecol Res 25:347–358
Xu H, Li Y (2006) Water-use strategy of three central Asian desert shrubs and their responses to rain pulse events. Plant Soil 28:5–17
Zhang D, Yin L, Pan B (2002) Biological and ecological characteristics of Tamarix L. and its effect on the ecological environment. Sci China 45:18–22
Acknowledgments
This research was part of the project “Harnessing the biodiversity of Mediterranean plants for mitigating the effects of climate change and desertification” coordinated by Prof. Riccardo Valentini and funded by the Italian Ministry of the Environment and Territory and Sea. We are grateful to the Laboratory of Microscopy of the Department for Innovation in Biological, Agro-food and Forest systems of the University of Tuscia. We would also like to thank Andrea Triani, Dr. Grazia Abbruzzese, Dr. Gabriele Guidolotti, Dr. Ettore D’Andrea, Dr. Victoria Dawalibi and Matilde Tamantini for their useful support and technical assistance.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Abou Jaoudé, R., de Dato, G. & De Angelis, P. Photosynthetic and wood anatomical responses of Tamarix africana Poiret to water level reduction after short-term fresh- and saline-water flooding. Ecol Res 27, 857–866 (2012). https://doi.org/10.1007/s11284-012-0963-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-012-0963-3