Abstract
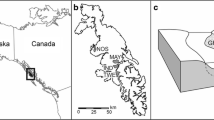
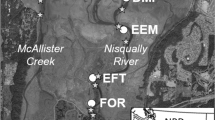
In coastal streams throughout the north Pacific region, spawning salmon (Oncorhynchus spp.) subsidize terrestrial communities with their nutrients and carcasses. We document the previously unreported composition and ecology of terrestrial invertebrates using salmon carcasses in forest habitats from two high salmon density watersheds in coastal British Columbia. From experimental placement of 186 carcasses, terrestrial Diptera-dominated salmon carcass decay (85.5% of carcasses). Overall, we recorded over 60 species from salmon carcasses, including saprophagous Diptera and Coleoptera (15 spp.), dipteran predators (eight spp.) and parasitoids (four spp.), and opportunistic predators, scavengers, and detritivores (24 spp.). Using stable isotopes of nitrogen and carbon, we reconstruct the dietary niches of select species relative to salmon muscle tissue and previously sampled non-salmon feeding invertebrate species. From comparisons across seasons, sampling locations and larval and adult life stages, we find evidence for a diet of salmon tissue in flies (Calliphora terraenovae and Dryomyza anilis), and beetles (Nicrophorus investigator and Anthobium fimetarium). The parasitic wasps Alysia alticola and Atractodes sp. had the highest levels of enrichment of all species, representing their larval diet of fly larvae that have fed on salmon carcasses 1 year prior to adult wasp sampling. Temporal and spatial isotopic variation in insect indicator species varies by species mobility and the pathway of salmon nutrient uptake. Cataloguing these associations may be useful for developing indices of intact salmon runs, bear foraging, and subsequent nutrient transfer in coastal watersheds.



Similar content being viewed by others
References
Anderson RS, Peck SB (1985) The carrion beetles of Canada and Alaska—Coleoptera: Silphidae and Agyrtidae. In: The insects and arachnids of Canada, part 13. Agriculture Canada, Ottawa, pp 1–121
Anderson WB, Polis GA (1998) Marine subsidies of island communities in the Gulf of California: evidence from stable carbon and nitrogen isotopes. Oikos 81:75–80. doi:10.2307/3546469
Ben-David M, Hanley TA, Klein DR, Schell DM (1997) Seasonal changes in diets of coastal and riverine mink: the role of spawning Pacific salmon. Can J Zool 75:803–811. doi:10.1139/z97-102
Ben-David M, Hanley TA, Schell DM (1998) Fertilization of terrestrial vegetation by spawning Pacific salmon: the role of flooding and predator activity. Oikos 83:47–55. doi:10.2307/3546545
Bilby RE, Fransen BR, Bisson PA (1996) Incorporation of nitrogen and carbon from spawning coho salmon into the trophic system of small streams: evidence from stable isotopes. Can J Fish Aquat Sci 53:164–173. doi:10.1139/cjfas-53-1-164
Borror DJ, Delong DM, Triplehorn CA (1981) An introduction to the study of insects, 5th edn. Saunders College Publishing, Philadelphia
Carabel S, Godínez-Domínguez E, Verísimo P, Fernández L, Freire J (2006) An assessment of sample processing methods for stable isotope analyses of marine food webs. J Exp Mar Biol Ecol 336:254–261. doi:10.1016/j.jembe.2006.06.001
Cederholm CJ, Kundze MD, Murota T, Sibatani A (1999) Pacific salmon carcasses: essential contributions of nutrients and energy for aquatic and terrestrial ecosystems. Fisheries 24:6–15. doi:10.1577/1548-8446(1999)024<0006:PSC>2.0.CO;2
Christie KS, Reimchen TE (2008) Presence of salmon increases passerine density on Pacific Northwest streams. Auk 125:51–59. doi:10.1525/auk.2008.125.1.51
Christie KS, Hocking MD, Reimchen TE (2008) Tracing salmon nutrients in riparian food webs: isotopic evidence in a ground-foraging passerine. Can J Zool 86:1317–1323
Darimont CT, Paquet PC, Reimchen TE (2008) Spawning salmon disrupt trophic coupling between wolves and ungulate prey in coastal British Columbia. BMC Ecol 8:14. doi:10.1186/1472-6785-8-14
Drake DC, Naiman RJ (2007) Reconstruction of Pacific salmon abundance from riparian tree-ring growth. Ecol Appl 17:1523–1542. doi:10.1890/06-1200.1
Ellers J, van Alphen JJM (2002) A trade-off between diapause duration and fitness in female parasitoids. Ecol Entomol 27:279–284. doi:10.1046/j.1365-2311.2002.00421.x
Finney BP, Gregory-Eaves I, Sweetman J, Douglas MSV, Smol JP (2000) Impacts of climate change and fishing on Pacific salmon abundance over the past 300 years. Science 290:795–799. doi:10.1126/science.290.5492.795
Furniss RL, Carolin VM (1977) Western forest insects. Miscellaneous publication no. 1339, U.S. Department of Agriculture, Forest Service, Washington, DC
Gende SM, Quinn TP (2004) The relative importance of prey density and social dominance in determining energy intake by bears feeding on Pacific salmon. Can J Zool 82:75–85. doi:10.1139/z03-226
Gende SM, Quinn TP, Willson MF (2001) Consumption choice by bears feeding on salmon. Oecologia 127:372–382. doi:10.1007/s004420000590
Gende SM, Edwards RT, Willson MF, Wipfli MS (2002) Pacific salmon in aquatic and terrestrial ecosystems. Bioscience 52:917–928. doi:10.1641/0006-3568(2002)052[0917:PSIAAT]2.0.CO;2
Gende SM, Quinn TP, Hilborn R, Hendry AP, Dickerson B (2004) Brown bears selectively kill salmon with high energy content but only in habitats that facilitate choice. Oikos 104:518–528. doi:10.1111/j.0030-1299.2004.12762.x
Green RN, Klinka K (1994) A field guide to site identification and interpretation for the Vancouver forest region. Research Branch of the Ministry of Forests, Victoria, British Columbia
Greenburg B (1991) Flies as forensic indicators. J Med Entomol 28:565–577
Gresh T, Lichatowich J, Schoonmaker P (2000) An estimation of historic and current levels of salmon production in the Northeast Pacific ecosystem: evidence of a nutrient deficit in the freshwater systems of the Pacific Northwest. Fisheries 25:15–21. doi:10.1577/1548-8446(2000)025<0015:AEOHAC>2.0.CO;2
Hatch MH (1953) The beetles of the Pacific Northwest. Part I: Introduction and Adephaga. University of Washington Publications in Biology 16(1). University of Washington Press, Seattle, Washington
Hatch MH (1957) The beetles of the Pacific Northwest. Part II: Staphiliniformia. University of Washington Publications in Biology 16(2). University of Washington Press, Seattle, Washington
Hilderbrand GV, Schwartz CC, Robbins CT, Jacoby ME, Hanley TA, Arthur SM, Servheen C (1999) The importance of meat, particularly salmon, to body size, population productivity, and conservation of North American brown bears. Can J Zool 77:132–138. doi:10.1139/cjz-77-1-132
Hobson KA, Sease JL, Merrick RL, Piatt JF (1997) Investigating trophic relationships of pinnipeds in Alaska and Washington using stable isotope ratios of nitrogen and carbon. Mar Mamm Sci 13:114–132. doi:10.1111/j.1748-7692.1997.tb00615.x
Hocking MD, Reimchen TE (2002) Salmon-derived nitrogen in terrestrial invertebrates from coniferous forests of the Pacific Northwest. BMC Ecol 2:4. doi:10.1186/1472-6785-2-4
Hocking MD, Reimchen TE (2006) Consumption and distribution of salmon (Oncorhynchus spp.) nutrients and energy by terrestrial flies. Can J Fish Aquat Sci 63:2076–2086. doi:10.1139/F06-110
Hocking MD, Ring RA, Reimchen TE (2006) Burying beetle Nicrophorus investigator reproduction on Pacific salmon carcasses. Ecol Entomol 31:5–12. doi:10.1111/j.0307-6946.2006.00747.x
Hocking MD, Darimont CT, Christie KS, Reimchen TE (2007) Niche variation in burying beetles (Nicrophorus spp.) associated with marine and terrestrial carrion. Can J Zool 85:437–442. doi:10.1139/Z07-016
Jauquet J, Pittman N, Heinis JA, Thompson S, Tatyama N, Cederholm J (2003) Observations of chum salmon consumption by wildlife and changes in water chemistry at Kennedy creek during 1997–2000. Am Fish Soc Symp 34:71–88
Kaeriyama M, Nakamura M, Edpalina R, Bower JR, Yamaguchi H, Walker RV, Myers KW (2004) Change in feeding ecology and trophic dynamics of Pacific salmon (Oncorhynchus spp.) in the central Gulf of Alaska in relation to climate events. Fish Oceanogr 13:197–207. doi:10.1111/j.1365-2419.2004.00286.x
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27. doi:10.1139/cjz-78-1-1
Kiljunen M, Grey J, Sinisalo T, Harrod C, Immonen H, Jones RI (2006) A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models. J Appl Ecol 43:1213–1222. doi:10.1111/j.1365-2664.2006.01224.x
Langellotto GA, Rosenheim JA, Williams MR (2006) Assessing trophic interactions in a guild of primary parasitoids and facultative hyperparasitoids: stable isotope analysis. Oecologia 150:291–299. doi:10.1007/s00442-006-0514-0
McConnaughey T, McRoy CP (1979) Food-web structure and fractionation of carbon isotopes in the Bering Sea. Mar Biol (Berl) 53:257–262. doi:10.1007/BF00952434
Meehan EP, Seminet-Reneau EE, Quinn TP (2005) Bear predation on Pacific salmon facilitates colonization of carcasses by fly maggots. Am Midl Nat 153:142–151. doi:10.1674/0003-0031(2005)153[0142:BPOPSF]2.0.CO;2
Pauly D, Trites AW, Capuli E, Christensen V (1998) Diet composition and trophic levels of marine mammals. J Mar Sci 55:467–481
Ponsard S, Arditi R (2000) What can stable isotopes (δ15N and δ13C) tell about the food web of soil macro-invertebrates? Ecology 81:852–864
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Quinn TP, Kinnison MT (1999) Size-selective and sex-selective predation by brown bears on sockeye salmon. Oecologia 121:273–282. doi:10.1007/s004420050929
Rainio J, Neimelä J (2003) Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers Conserv 12:487–506. doi:10.1023/A:1022412617568
Reimchen TE (1994) Further studies of black bear and chum salmon in stream and estuarine habitats at Bag Harbour, Gwaii Haanas. Canadian Parks Service
Reimchen TE (2000) Some ecological and evolutionary aspects of bear-salmon interactions in coastal British Columbia. Can J Zool 78:448–457. doi:10.1139/cjz-78-3-448
Reimchen TE, Mathewson D, Hocking MD, Moran J, Harris D (2003) Isotopic evidence for enrichment of salmon-derived nutrients in vegetation, soil, and insects in riparian zones in coastal British Columbia. Am Fish Soc Symp 34:59–69
Spellerberg IF (1993) Monitoring ecological change. Cambridge University Press, Cambridge, UK
Stockner JG (2003) Nutrients in salmonid ecosystems: sustaining production and biodiversity. Am Fish Soc, Bethesda, Maryland
Tallamy DW, Pesek JD (1996) Carbon isotope signatures of elytra reflect larval diet in Luperine Rootworms (Coleoptera: Chrysomelidae). Environ Entomol 25:1167–1172
Tibbets TM, Wheeless LA, Martínez del Rio C (2008) Isotopic enrichment without change in diet: an ontogenetic shift in δ15N during insect metamorphosis. Funct Ecol 22:109–113
Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136:169–182. doi:10.1007/s00442-003-1270-z
Wilkinson CE, Hocking MD, Reimchen TE (2005) Uptake of salmon-derived nitrogen by mosses and liverworts in Coastal British Columbia. Oikos 108:85–98. doi:10.1111/j.0030-1299.2005.13277.x
Willson MF, Halupka KC (1995) Anadromous fish as keystone species in vertebrate communities. Conserv Biol 9:489–497. doi:10.1046/j.1523-1739.1995.09030489.x
Wipfli MS, Hudson J, Caouette J (1998) Influence of salmon carcasses on stream productivity: response of biofilm and benthic macroinvertebrates in southeastern Alaska, USA. Can J Fish Aquat Sci 55:1503–1511. doi:10.1139/cjfas-55-6-1503
Yanai S, Kochi K (2005) Effects of salmon carcasses on experimental stream ecosystems in Hokkaido, Japan. Ecol Res 20:471–480. doi:10.1007/s11284-005-0056-7
Zhang YX, Negishi JN, Richardson JS, Kolodziejczyk R (2003) Impacts of marine-derived nutrients on stream ecosystem functioning. Proc R Soc Lond 270:2117–2123. doi:10.1098/rspb.2003.2478
Acknowledgments
Thanks to C. Brinkmeier, K. Christie, C. Darimont, B. Foster, T. Gladstone, R. Hocking, S. Hocking, R. Johnson, L. Jorgenson, K. Petkau, C. Wilkinson, B. Windsor, D. Windsor, and M. Windsor, as well as the Raincoast Conservation Society and the Heiltsuk First Nations for field support. Thanks to all invertebrate taxonomists for identifications, M. Durban and J. Wray from Blue Fjord Charters, E. Darling, W. Palen and Z. Lindo for review comments, B. Hawkins at the University of Victoria, J. Reynolds at Simon Fraser University, and M. Stocki for stable isotope analysis at the University of Saskatchewan. Financial support was provided by grants to T. Reimchen from the David Suzuki Foundation and the Natural Sciences and Engineering Research Council of Canada (NSERC), and from Industrial Postgraduate scholarships to M. Hocking.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hocking, M.D., Ring, R.A. & Reimchen, T.E. The ecology of terrestrial invertebrates on Pacific salmon carcasses. Ecol Res 24, 1091–1100 (2009). https://doi.org/10.1007/s11284-009-0586-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-009-0586-5