Abstract
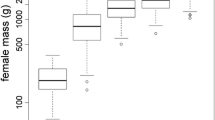
Viability selection and fecundity of size-related traits has been demonstrated to be strong in vertebrates. In small mammals, both offspring and adult size are important for viability and fecundity, respectively. We studied the role of early phenotypic selection on size attributes and female fecundity in the leaf-eared mouse (Phyllotis darwini). Our results support that larger females produce more offspring, and since the likelihood of attaining adulthood is similar for different sizes of the females, those larger females also produce more offspring that attain sexual maturity. From the offspring perspective, larger pups at birth have significantly more probability of attaining sexual maturity. However, weaning mass and growth rate did not show any differential survival. Our study suggests that early selection could be important and could prevent further episodes of selection by early culling of the distribution of sizes, and that “effective” fecundity is strongly dependent on the size of the female.





Similar content being viewed by others
References
Bacigalupe LD, Bozinovic F (2002) Design, limitations and sustained metabolic rate: lessons from small mammals. J Exp Biol 205:2963–2970
Blumberg MS, Sokoloff G (1997) Dynamics of brown fat thermogenesis in week-old rats: evidence of relative stability during moderate cold exposure. Physiol Zool 70:324–330
Browne MW (1984) Asymptotically distribution-free methods for the analysis of covariance structures. Br J Math Stat Psychol 37:62–83
Du W, Xiang J, Shine R (2005) Does body volume constrain reproductive output in lizards? Biol Lett 1:98–100. doi:10.1098/rsbl.2004.0268
Endler JA (1986) Natural selection in the wild. Monographs in population biology. Princeton University Press, New Jersey
Fleming TH, Rausher RJ (1978) On the evolution of litter size in Peromyscus maniculatus. Evolution Int J Org Evol 32:45–55. doi:10.2307/2407409
Fournier F, Thomas DW, Garland T (1999) A test of two hypotheses explaining the seasonality of reproduction in temperate mammals. Funct Ecol 13:523–529. doi:10.1046/j.1365-2435.1999.00342.x
Fox CW (2000) Natural selection on seed-beetle egg size in nature and the laboratory: variation among environments. Ecology 81:3029–3035
Fox CW, Czesak ME (2000) Evolutionary ecology of progeny size in arthropods. Annu Rev Entomol 45:341–369. doi:10.1146/annurev.ento.45.1.341
Hayes JP, O’Connor CSO (1999) Natural selection on thermogenic capacity of high-altitude deer mice. Evolution Int J Org Evol 53:1280–1287. doi:10.2307/2640830
Janzen FJ (1993) An experimental analysis of natural selection on body size of hatchling turtles. Ecology 74:332–341. doi:10.2307/1939296
Janzen FJ, Stern HS (1998) Logistic regression for empirical studies of multivariate selection. Evolution Int J Org Evol 52:1564–1571. doi:10.2307/2411330
Kenagy GJ, Barnes BM (1988) Seasonal reproductive patterns in four coexisting rodents species from the Cascade Mountains, Washington. J Mammal 69:274–292. doi:10.2307/1381378
Kenagy GJ, Masman D, Sharbaugh SM, Nagy KA (1990) Energy expenditure during lactation in relation to litter size in free-living golden-mantled ground squirrels. J Anim Ecol 59:73–88. doi:10.2307/5159
Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, Gibert P, Beerli P (2001) The strength of phenotypic selection in natural populations. Am Nat 157:245–261. doi:10.1086/319193
Koskela E, Mappes T, Ylonen H (1999) Experimental manipulation of breeding density and litter size: effects on reproductive success in the bank vole. J Anim Ecol 68:513–521. doi:10.1046/j.1365-2656.1999.00308.x
Lynch M, Walsh B (1998) Genetics and analysis of quantitative traits. Sinauer, Sunderland
Manly BF (1998) Randomization, bootstrap and Monte Carlo methods in biology. Chapman and Hall, London
Mappes T, Koskela E (2004) Genetic basis of the trade-off between offspring number and quality in the bank vole. Evolution Int J Org Evol 58:645–650
McAdam AG, Boutin S (2003) Variation in viability selection among cohorts of juvenile red squirrels (Tamiasciurus hudsonicus). Evolution Int J Org Evol 57:1689–1697
Millar JS (1978) Energetics of reproduction in Peromyscus leucopus: the cost of lactation. Ecology 59:1055–1061. doi:10.2307/1938558
Morris DW (1985) Natural selection for reproductive optima. Oikos 45:290–293. doi:10.2307/3565719
Morris DW (1996) State-dependent life histories, Mountford’s hypothesis and the evolution of brood size. J Anim Ecol 65:43–51. doi:10.2307/5698
Myers P, Master LL (1983) Reproduction by Peromyscus maniculatus: size and compromise. J Mammal 64:1–18. doi:10.2307/1380746
Nespolo RF, Bacigalupe LD, Bozinovic F (2003) Heritability of energetics in a wild mammal, the leaf-eared mouse (Phyllotis darwini). Evolution Int J Org Evol 57:1679–1688
Nespolo RF, Bustamante DM, Bacigalupe LD, Bozinovic F (2005) Quantitative genetics of bioenergetics and growth-related traits in the wild mammal, Phyllotis darwini. Evolution Int J Org Evol 59:1829–1837
Oksanen TA, Jonsson P, Koskela E, Mappes T (2001) Optimal allocation of reproductive effort: manipulation of offspring number and size in the bank vole. Proc R Soc Lond B Biol Sci 268:661–666. doi:10.1098/rspb.2000.1409
Oksanen TA, Koskela E, Mappes T (2002) Hormonal manipulation of offspring number: maternal effort and reproductive costs. Evolution Int J Org Evol 56:1530–1537
Oksanen TA, Jokinen I, Koskela E, Mappes T, Vilpas H (2003) Manipulation of offspring number and size: benefits of large body size at birth depend upon the rearing environment. J Anim Ecol 72:321–330. doi:10.1046/j.1365-2656.2003.00703.x
Oksanen TA, Koivula M, Koskela E, Mappes T (2007) The cost of reproduction induced by body size at birth and breeding density. Evolution Int J Org Evol 61:2822–2831. doi:10.1111/j.1558-5646.2007.00245.x
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Rogowitz GL (1998) Limits to milk flow and energy allocation during lactation of the hispid cotton rat (Sigmodon hispidus). Physiol Zool 71:312–320
Rogowitz GL, McClure PA (1995) Energy export and offspring growth during lactation in cotton rats (Sigmodon hispidus). Funct Ecol 9:143–150. doi:10.2307/2390558
Ruusila V, Ermala A, Hyvarinen H (2000) Costs of reproduction in introduced female Canadian beavers (Castor canadensis). J Zool (Lond) 252:79–82. doi:10.1111/j.1469-7998.2000.tb00822.x
Schluter D (1988) Estimating the form of natural selection on a quantitative trait. Evolution Int J Org Evol 42:849–861. doi:10.2307/2408904
Sheldon BC, Kruuk LEB, Merila J (2003) Natural selection and inheritance of breeding time and clutch size in the collared flycatcher. Evolution Int J Org Evol 57:406–420
Shipley B (2000) Cause and correlation in biology. Cambridge University Press, Cambridge
Sikes RS (1995) Costs of lactation and optimal litter size in northern grasshopper mice (Onychomys leucogaster). J Mammal 76:348–357. doi:10.2307/1382346
Sikes RS (1998) Tradeoffs between quality of offspring and litter size: differences do not persist into adulthood. J Mammal 79:1143–1151. doi:10.2307/1383005
Sinervo B (1990) Evolution of thermal physiology and growth rate between populations of the western fence lizard (Sceloporus occidentalis). Oecologia 83:228–237. doi:10.1007/BF00317757
Sinervo B, Licht P (1991) Proximate constraints on the evolution of egg size, number and total clutch mass in lizards. Science 191:1300–1302. doi:10.1126/science.252.5010.1300
Steppan SJ (1998) Phylogenetic relationships and species limits within Phyllotis (Rodentia: Sigmodontinae): concordance between Mtdna sequence and morphology. J Mammal 79:573–593. doi:10.2307/1382988
Svensson E, Sinervo B (2000) Experimental excursions on adaptive landscapes: density-dependent selection on egg size. Evolution Int J Org Evol 54:1396–1403
Webb DR, Porter WP, McClure PA (1990) Development of insulation in juvenile rodents: functional compromise in insulation. Funct Ecol 4:251–256. doi:10.2307/2389344
Young KV, Brodie ED, Brodie EDI (2004) How the horned lizard got its horns. Science 304:65. doi:10.1126/science.1094790
Acknowledgments
This study was funded by a FONDECYT grant 3030032. We thank Francisco Bozinovic for laboratory facilities and critical revision of the first draft and Diego Bustamante for logistic help.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nespolo, R.F., Bacigalupe, L.D. Viability selection on early body mass and the effect of female body size on fecundity: a study on the leaf-eared mouse Phyllotis darwini . Ecol Res 24, 997–1002 (2009). https://doi.org/10.1007/s11284-008-0570-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-008-0570-5