Abstract
Objectives
To explore the effectiveness of magnetic resonance image (MRI)-based biomarkers for identifying benign and malignant parotid tumors via diagnostic model analysis.
Methods
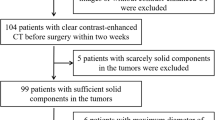
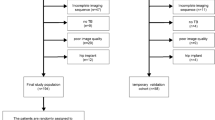
This retrospective study included 109 patients (development cohort and validation cohort) who underwent MRI preoperatively, including T1- and T2-weighted images. Parameters based on 2D or 3D texture analysis were extracted from tumor lesions by MaZda software, fisher discriminant and bootstrap method were used to perform parameter reduction, diagnostic models with the selected biomarkers were established along with clinical data, model performance (discrimination and calibration) was furtherly evaluated by internal and external validation, decision curve analysis was applied to measure the improvement of clinical benefits.
Results
S(5,5) Entrop, S(0,1) ASM, WavEnHH (s-4), S(1,1,0) Entropy and Perc.10% were significantly associated with the pathological diagnosis of parotid tumor (benign versus malignancy), when adding these biomarkers to the regression analysis, model performance significantly improved in the development cohort (likelihood-ratio-test; p < 0.05, with an increase of AUC from 0.72 (reference model) to 0.85), and these results were maintained in a small external validation cohort. Decision curve analysis indicated that clinical benefit was greater with the application of MRI-based biomarkers.
Conclusions
MRI-based texture analysis is proven to be an effective tool in differentiating benign and malignant parotid tumors, preoperative diagnosis was improved with the selected biomarkers compared to the reference model.



Similar content being viewed by others
References
Pinkston JA, Cole P. Incidence rates of salivary gland tumors: results from a population-based study. Otolaryngol Head Neck Surg. 1999;120(6):834–40.
Okahara M, Kiyosue H, Hori Y, Matsumoto A, Mori H, Yokoyama S. Parotid tumors: MR imaging with pathological correlation. Eur Radiol. 2003;13(6):L25–33.
Woods JE, Chong GC, Beahrs OH. Experience with 1,360 primary parotid tumors. Am J Surg. 1975;130(4):460–2.
Som PM, Curtin H. Head and Neck Imaging, vol. 2. 5th ed. Anatomy and pathology of the salivary glands. Mosby, Missouri. 2011;2449–610.
Christe A, Waldherr C, Hallett R, Zbären P, Thoeny H. MR imaging of parotid tumors: typical lesion characteristics in MR imaging improve discrimination between benign and malignant disease. AJNR Am J Neuroradiol. 2011;32(7):1202–7.
Faheem MH, Shady S, Refaat MM. Role of magnetic resonance imaging (MRI) including diffusion weighted images (DWIs) in assessment of parotid gland masses with histopathological correlation. J Radiol Nucl Med. 2018;49(2):368–73.
Yu H. Automated segmentation of head and neck cancer using texture analysis with co-registered PET/CT images. 2010. http://hdl.handle.net/1807/24920. Accessed 2 Sep 2010.
Wong AJ, Kanwar A, Mohamed AS, Fuller CD. Radiomics in head and neck cancer: from exploration to application. Transl Cancer Res. 2016;5(4):371.
Fruehwald-Pallamar J, Czerny C, Holzer-Fruehwald L, Nemec SF, Mueller-Mang C, Weber M, et al. Texture-based and diffusion-weighted discrimination of parotid gland lesions on MR images at 3.0 Tesla. NMR Biomed. 2013;26(11):1372–9.
Mikaszewski B, Markiet K, Smugała A, Stodulski D, Szurowska E, Stankiewicz C. Diffusion-weighted MRI in the differential diagnosis of parotid malignancies and pleomorphic adenomas: can the accuracy of dynamic MRI be enhanced? NMR Biomed. 2017;124(1):95–103.
Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–73.
Szczypiński PM, Strzelecki M, Materka A, Klepaczko A. MaZda—a software package for image texture analysis. Comput Methods Programs Biomed. 2009;94(1):66–76.
Hajek M, Dezortova M, Materka A, Lerski R. Texture analysis for magnetic resonance imaging. Med4publishing, Neherovská. 2006.
Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, et al. Radiomics: the process and the challenges. Magn Reson Imaging. 2012;30(9):1234–48.
Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87.
Lu SS, Kim SJ, Kim N, Kim HS, Choi CG, Lim YM. Histogram analysis of apparent diffusion coefficient maps for differentiating primary CNS lymphomas from tumefactive demyelinating lesions. AJR Am J Roentgenol. 2015;204(4):827–34.
Just N. Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer. 2014;111(12):2205–13.
Joe V, Westesson P. Tumors of the parotid gland: MR imaging characteristics of various histologic types. AJR Am J Roentgenol. 1994;163(2):433–8.
Triki R, Abdelkafi M, Jerbi S, Zayani O, Hamida NB, Rhouma KB, et al. Magnetic resonance imaging contribution in the characterization of parotid tumors. European Society of Radiology. 2017. https://doi.org/10.1594/ecr2017/C-1004.
Zwanenburg A, Leger S, Vallières M, Löck S. Image biomarker standardisation initiative. arXiv preprint arXiv: 161207003. 2016.
Zhang W, Zuo Z, Huang X, Jin G, Su D. Value of diffusion-weighted imaging combined with susceptibility-weighted imaging in differentiating benign from malignant parotid gland lesions. Med Sci Monit. 2018;24:4610.
Zayed N, Elnemr HA. Statistical analysis of haralick texture features to discriminate lung abnormalities. Int J Biomed Imaging. 2015. https://doi.org/10.1155/2015/267807.
Michael U, Aktam A, Andrew L. Guest editorial: wavelets in medical imaging. IEEE Trans Med Imaging. 2003;22(3):285–8. https://doi.org/10.1109/TMI.2003.809638. Accessed 21 May 2003.
Yao J, Chen J, Chow C. Breast tumor analysis in dynamic contrast enhanced MRI using texture features and wavelet transform. IEEE J Sel Top Signal Process. 2009;3(1):94–100.
Sollini M, Antunovic L, Chiti A, Kirienko M. Towards clinical application of image mining: a systematic review on artificial intelligence and radiomics. Eur J Nucl Med Mol Imaging. 2019;46(13):2656–72.
Sakamoto M, Iikubo M, Kojima I, Sasano T, Mugikura S, Murata T, et al. Diagnostic value of capsule-like rim enhancement on magnetic resonance imaging for distinguishing malignant from benign parotid tumours. Int J Oral Maxillofac Surg. 2014;43(9):1035–41.
Freling N, Molenaar W, Vermey A, Mooyaart E, Panders A, Annyas A, et al. Malignant parotid tumors: clinical use of MR imaging and histologic correlation. Radiol. 1992;185(3):691–6.
Shao S, Mao N, Liu W, Cui J, Xue X, Cheng J, et al. Epithelial salivary gland tumors: Utility of radiomics analysis based on diffusion-weighted imaging for differentiation of benign from malignant tumors. J Xray Sci Technol. 2020;1:1–12.
Fruehwald-Pallamar J, Hesselink J, Mafee M, Holzer-Fruehwald L, Czerny C, Mayerhoefer M, et al. Texture-based analysis of 100 MR examinations of head and neck tumors–is it possible to discriminate between benign and malignant masses in a multicenter trial? RöFo-Fortschritte Gebiet Röntgenstrahlen bildgebenden Verfahren. 2016;188(2):195–202.
King AD, Thoeny HC. Functional MRI for the prediction of treatment response in head and neck squamous cell carcinoma: potential and limitations. Cancer Imaging. 2016;16(1):23.
Fujima N, Sakashita T, Homma A, Shimizu Y, Yoshida A, Harada T, et al. Advanced diffusion models in head and neck squamous cell carcinoma patients: goodness of fit, relationships among diffusion parameters and comparison with dynamic contrast-enhanced perfusion. Magn Reson Imaging. 2017;36:16–23.
Swartz JD, Rothman MI, Marlowe FI, Berger AS. MR imaging of parotid mass lesions: attempts at histopathologic differentiation. J Comput Assist Tomogr. 1989;13(5):789–96.
Acknowledgements
We thank Editage (www.editage.cn) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the Beijing Natural Science Foundation of Beijing, China (B420803).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Animal rights statements
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., Zheng, J., Zhao, J. et al. Magnetic resonance image biomarkers improve differentiation of benign and malignant parotid tumors through diagnostic model analysis. Oral Radiol 37, 658–668 (2021). https://doi.org/10.1007/s11282-020-00504-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11282-020-00504-4