Abstract
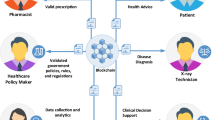
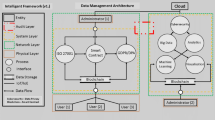
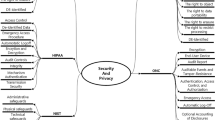
Information management is Silos of the impending conditions across the sectors. Predominantly in the case of the clinical trials management, one of the critical challenges is about the information silos among the various stakeholder’s integral to the process. Processing the information over real-time and ensuring there is a holistic system in place shall help in improving the quality of collaboration and integration. The objective of this research article is to assess the feasibility of blockchain implementation for collaborative clinical trial auditing and to propose a blockchain-based solution that can help in improving the quality of solutions structure practised, leading to sustainable ways of handling the clinical trial auditing. The proposed model develops grid-based blocks for each of the phases of clinical trials, forming a comprehensive block network to be shared among the consortium handling the data. The proposed framework has a significant scope of security enhancement and customization that can help in shaping the systematic improvement to the information systems of clinical trials. The research methodology adopts the blockchain framework, which has been discussed in the paper. The consensus protocol used is Practical Byzantine Fault Tolerance (PBFT). The experimental study was conducted using simulation to verify the performance with regard to different performance metrics and attacks. The results showed that the proposed blockchain framework provides improved transaction throughput, reduced latency, and enhanced scalability, as compared to other existing models.





Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References
McCoy, C. (2006). Writing clinical research protocols: Ethical considerations. Clinical Laboratory Science, 19(1), 60.
Draper, H., Wilson, S., Flanagan, S., & Ives, J. (2009). Offering payments, reimbursement and incentives to patients and family doctors to encourage participation in research. Family Practice, 26(3), 231–238.
Dickert, N. W., Halpern, S. D., & Butler, J. (2013). Incentivizing recruitment and retention to address enrollment challenges in clinical research. Circulation: Cardiovascular Quality and Outcomes, 6(3), 367–370.
https://www.nccn.org/patients/resources/clinical_trials/phases.aspx.
https://www.dataversity.net/can-blockchain-eliminate-the-data-silo/#.
Brooks, R., Skjellum, A., Worley, C., Obeid J. S., Lenert, L. (2018). Scrybe: A blockchain ledger for clinical trials. http://blockchain.ieee.org/images/files/images/clinicaltrialsforum-2018/Clemson_WhitePaper.pdf (18.235.102.153). (Accessed 15 Dec 2018).
Irving, G., Holden, J. (2016). How blockchain-timestamped protocols could improve the trustworthiness of medical science. F1000research.
Ramachandran, A., Kantarcioglu, M. (2017). Using blockchain and smart contracts for secure data provenance management. arXiv:1709.10 000v1 [cs.CR].
Jiang, S., Cao, J., Wu, H., Yang, Y., Ma, M., He, J. (2018). BlocHIE: A BLOCkchain-based platform for healthcare information exchange. In IEEE International conference on smart computing.
Zhang, P., White, J., Schmidt, D. C., Lenz, G., & Rosenbloom, S. T. (2018). FHIRChain: Applying blockchain to securely and scalably share clinical data. Computational and Structural Biotechnology Journal, 16, 267–278.
Choudhury, O., Fairoza, N., Sylla, I., Das, A. (2019). A blockchain framework for managing and monitoring data in multi-site clinical trials. arXiv:1902.03975 [cs.DB], pp. 1–13.
Zhuang, Y., Sheets, L., Shae, Z., Tsai, J. J. P., Shyu, C.-R. (2018). Applying blockchain technology for health information exchange and persistent monitoring for clinical trials. In AMIA annual symposium proceedings archive.
Maslove, D. M., Klein, J., Brohman, K., Martin, P. (2018). Using blockchain technology to manage clinical trials data: A proof-of-concept study. In JMIR Medical Informatics.
Malik, S. et al., (2021). Blockchain in clinical trials. In Opportunities and challenges for blockchain technology in autonomous vehicles. IGI Global, pp. 278–292.
Yli-Huumo, J., Ko, D., Choi, S., Park, S., & Smolander, K. (2016). Where is current research on blockchain technology?—a systematic review. PLoS ONE, 11(10), e0163477.
Boulos, M. N. K., Wilson, J. T., & Clauson, K. A. (2018). Geospatial blockchain: promises, challenges, and scenarios in health and healthcare. 25.
Chen, Y., Ding, S., Xu, Z., Zheng, H., & Yang, S. (2019). Blockchain-based medical records secure storage and medical service framework. Journal of medical systems., 43(1), 5.
Kuo, T. T., Kim, H. E., & Ohno-Machado, L. (2017). Blockchain distributed ledger technologies for biomedical and health care applications. Journal of the American Medical Informatics Association., 24(6), 1211–1220.
Griggs, K. N., Ossipova, O., Kohlios, C. P., Baccarini, A. N., Howson, E. A., & Hayajneh, T. (2018). Healthcare blockchain system using smart contracts for secure automated remote patient monitoring. Journal of medical systems., 42(7), 130.
Nugent, T., Upton, D., Cimpoesu, M. (2016). Improving data transparency in clinical trials using blockchain smart contracts. F1000Research. 5.
Park, J. S., Youn, T. Y., Kim, H. B., Rhee, K. H., & Shin, S. U. (2018). Smart contract-based review system for an IoT data marketplace. Sensors, 18(10), 3577.
Acknowledgements
I would like to express my sincere gratitude to Professor Zhaoshun Wang and to the School of Computer and Communication Engineering, University of Science and Technology, Beijing.
Funding
No funding is involved in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Conflict of interest is not applicable in this work.
Ethical Approval
No participation of humans takes place in this implementation process.
Consent to Participate
No participation of humans takes place in this implementation process.
Human and Animal Rights
No violation of Human and Animal Rights is involved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdu, N.A.A., Wang, Z. Blockchain Framework for Collaborative Clinical Trials Auditing. Wireless Pers Commun 132, 39–65 (2023). https://doi.org/10.1007/s11277-023-10575-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11277-023-10575-1