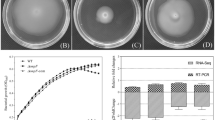
Abstract
The protective role of superoxide dismutase (Sod) against oxidative stress, resulting from the common antibiotic pathway of action, has been studied in the wild type and mutant strains of swarmer Pseudomonas aeruginosa, lacking Cytosolic Mn-Sod (sodM), Fe-Sod (sodB) or both Sods (sodMB).
Our results showed that inactivation of sodB genes leads to significant motility defects and tolerance to meropenem. This resistance is correlated with a greater membrane unsaturation as well as an effective intervention of Mn-Sod isoform, in antibiotic tolerance.
Moreover, loss of Mn-Sod in sodM mutant, leads to polymixin intolerance and is correlated with membrane unsaturation. Effectivelty, sodM mutant showed an enhanced swarming motility and a conserved rhamnolipid production. Whereas, in the double mutant sodMB, ciprofloxacin tolerance would be linked to an increase in the percentage of saturated fatty acids in the membrane, even in the absence of superoxide dismutase activity.
The overall results showed that Mn-Sod has a protective role in the tolerance to antibiotics, in swarmer P.aeruginosa strain. It has been further shown that Sod intervention in antibiotic tolerance is through change in membrane fatty acid composition.
Graphic abstract





Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aloui A, Mihoub M, Sethom MM, Chatti A, Feki M, Kaabachi N, Landoulsi A (2010) Effects of dam and/or seqA mutations on the fatty acid and phospholipid membrane composition of Salmonella enterica serovar Typhimurium. Foodborne Pathog Dis 7(5):573–583
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917.
Chen H et al (2020) In vitro and in vivo effects of the polymyxin-vorinostat combination therapy against multidrug-resistant Gram-negative pathogens. Microb Drug Resist 269:1108–1119. https://doi.org/10.1089/mdr.2019.0309
Da Cruz N, Waleska S et al (2021) Oxidative stress response in Pseudomonas aeruginosa. Pathogens 10:9: 1187. https://doi.org/10.3390/pathogens10091187
Davenport PW, Griffin JL, Welch M (2015) Quorum sensing is accompanied by global metabolic changes in the opportunistic human pathogen Pseudomonas aeruginosa. J Bacteriol 197:2072–2082. https://doi.org/10.1128/JB.02557-14
Dwyer Daniel J, Michael A, Kohanski, Collins JJ (2009) Role of reactive oxygen species in antibiotic action and resistance. Current opinion in microbiology.12.5. 482–489. https://doi.org/10.1016/j.mib.2009.06.018
Fee JA (1991) Regulation of sod genes in Escherichia coli: relevance to superoxide dismutase function. Mol Microbiol 5(11):2599–2610
Gales AC, Jones RN, Gordon KA, Sader HS, Wilke WW, Beach ML, Pfaller MA, Doern GV, SENTRY Study Group Latin America T (2000) Activity and spectrum of 22 antimicrobial agents tested against urinary tract infection pathogens in hospitalized patients in Latin America: report from the second year of the SENTRY antimicrobial surveillance program (1998). J Antimicrob Chemother 45(3):295–303
García-Contreras R et al (2020) Rhamnolipids stabilize quorum sensing mediated cooperation in Pseudomonas aeruginosa. FEMS Microbiol Lett 367:10: fnaa080. https://doi.org/10.1093/femsle/fnaa080
Ghorbal SK, Chatti A, Sethom MM, Maalej L, Mihoub M, Kefacha S, Feki M, Landoulsi A, Hassen A (2013) Changes in membrane fatty acid composition of Pseudomonas aeruginosa in response to UV-C radiations. Curr Microbiol 67:112–117
Grant SS, Kaufmann BB, Chand NS, Haseley N, Hung DT (2012) Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proceedings of the National Academy of Sciences. 109(30): 12147–12152
Hanini R et al (2017) Role of sod gene in response to static magnetic fields in Pseudomonas aeruginosa. Curr Microbiol 4:8: 930–937. https://doi.org/10.1007/s00284-017-1264-4
Hassett DJ et al (1999) Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol Microbiol 345:1082–1093. https://doi.org/10.1046/j.1365-2958.1999.01672.x
Heindorf M et al (2014) Impact of Acinetobacter baumannii superoxide dismutase on motility, virulence, oxidative stress resistance and susceptibility to antibiotics. PLoS ONE. https://doi.org/10.1371/journal.pone.0101033. .9.7: e101033
Igbinosa IH, Etinosa OI (2015) The Pseudomonads as a versatile opportunistic pathogen in the environment. The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs. Formatex Research Center Badajoz, Spain. 822–831
Imlay JA (2013) The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11(7):443–454
Kiratisin P, Tucker KD, Passador L (2002) LasR, a transcriptional activator of Pseudomonas aeruginosa virulence genes, functions as a multimer. J Bacteriol 184(17):4912–4919
Latif IA (2018) Swarming motility patterns of Pseudomonas aeruginosa isolated from Otitis media. Tikrit J Pure Sci 20(4):26–29
Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, Jeffers AK (2005) The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-γ-mediated macrophage killing. J Immunol 175(11):7512–7518
Liang Z, Nilsson M, Kragh KN, Hedal I, Alcàcer-Almansa J, Kiilerich RO, …, Tolker-Nielsen T (2023) The role of individual exopolysaccharides in antibiotic tolerance of Pseudomonas aeruginosa aggregates. Front Microbiol 14:1187708
Liaw SJ, Lai HC, Wang WB (2004) Modulation of swarming and virulence by fatty acids through the RsbA protein in Proteus mirabilis. Infect Immun 72(12):6836–6845
Lima OC, Larcher G, Vandeputte P, Lebouil A, Chabasse D, Simoneau P, Bouchara JP (2007) Molecular cloning and biochemical characterization of a Cu, Zn-superoxide dismutase from Scedosporium Apiospermum. Microbes Infect 9(5):558–565
Martins D et al (2018) Superoxide dismutase activity confers (p) ppgpp-mediated antibiotic tolerance to stationary-phase Pseudomonas aeruginosa. Proc Natl Acad Sci 115(39):9797–9802. https://doi.org/10.1073/pnas.1804525115
Martins D, McKay GA, English AM, Nguyen D (2020) Sublethal paraquat confers multidrug tolerance in Pseudomonas aeruginosa by inducing superoxide dismutase activity and lowering envelope permeability. Front Microbiol 11:576708
Maurya R, Namdeo M (2021) Superoxide dismutase: a key enzyme for the survival of intracellular pathogens in host. Reactive Oxygen Species
Meliani A, Bensoltane A (2018) Chemotaxis Behavior of Pseudomonas species and Biodegradation of pollutants. Sustainable Agric Reviews 31:483–507. https://doi.org/10.1007/978-3-319-94232-2_10
Miao L, Clair DKS (2009) Regulation of superoxide dismutase genes: implications in Disease. Free Radic Biol Med 47(4):344–356
Moon YJ, Kim SI, Chung YH (2012) Sensing and responding to UV-A in cyanobacteria. Int J Mol Sci 13(12):16303–16332
Nassar O, Desouky SE, El-Sherbiny GM, Abu-Elghait M (2022) Correlation between phenotypic virulence traits and antibiotic resistance in Pseudomonas aeruginosa clinical isolates. Microb Pathog 162:105339
Overhage J et al (2008) Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. 2671–2679. https://doi.org/10.1128/JB.01659-07
Pinzon NM, Lu-Kwang JU (2009) Improved detection of rhamnolipid production using agar plates containing methylene blue and cetyl trimethylammonium bromide. Biotechnol Lett 3110:1583–1588. https://doi.org/10.1007/s10529-009-0049-7
Rada Balázs (2017) Interactions between neutrophils and Pseudomonas aeruginosa in cystic fibrosis. Pathogens. https://doi.org/10.3390/pathogens6010010. 6.1: 10
Salma KB, Lobna M, Sana K, Kalthoum C, Imene O, Abdelwaheb C (2016) Antioxidant enzymes expression in Pseudomonas aeruginosa exposed to UV-C radiation. J Basic Microbiol 56(7):736–740.
Sampson TR, Liu X, Schroeder MR, Kraft CS, Burd EM, Weiss DS (2012) Rapid killing of Acinetobacter baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrob Agents Chemother 56(11):5642–5649
Sikkema JAN, Jan A, de Bont, Bert Poolman (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Reviews 59 2201–222. https://doi.org/10.1128/mr.59.2.201-222.1995
Tremblay J, Déziel E (2010) Gene expression in Pseudomonas aeruginosa swarming motility. BMC Genomics 11(1):1–15
Valentin JD, Straub H, Pietsch F, Lemare M, Ahrens CH, Schreiber F, …, Ren Q (2022) Role of the flagellar hook in the structural development and antibiotic tolerance of Pseudomonas aeruginosa biofilms. ISME J 16(4):1176–1186
Van Acker H, Gielis J, Acke M, Cools F, Cos P, Coenye T (2016) The role of reactive oxygen species in antibiotic-induced cell death in Burkholderia cepacia complex bacteria. PLoS ONE 11(7):e0159837
Wayne P (2014) CLSI performance standard of antimicrobial susceptibility testing: twenty-fourth international supplement. CLSI Document M100-S24, Clinical and Laboratory Standard Institute. 34(1):50–106
Xavier JB, Kim W, Foster KR (2011) A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa Xavier – 2010 - Molecular Microbiology - Wiley Online Library. Mol Microbiol 79:166–179. https://doi.org/10.1111/j.1365-2958.2010.07436.x
Yeung AT, Parayno A, Hancock RE (2012) Mucin promotes rapid surface motility in Pseudomonas aeruginosa. MBio 3(3):e00073–e00012
Zhao Y, Hindorff LA, Chuang A, Monroe-Augustus M, Lyristis M, Harrison ML, …, Bennett GN (2003) Expression of a cloned cyclopropane fatty acid synthase gene reduces solvent formation in Clostridium acetobutylicum ATCC 824. 69(5):2831–2841Applied and environmental microbiology
Acknowledgements
The authors are grateful to Professor Moncef Feki of la Rabta-hospital biochemistry department in Tunisia for ensuring the Gaz chromatography analysis.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben Ghorbal, S.K., Maalej, L., Ouzari, IH. et al. Implication of Mn-cofactored superoxide dismutase in the tolerance of swarmer Pseudomonas aeruginosa to polymixin, ciprofloxacin and meropenem antibiotics. World J Microbiol Biotechnol 39, 347 (2023). https://doi.org/10.1007/s11274-023-03801-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03801-2