Abstract
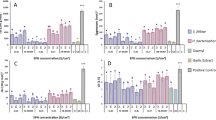
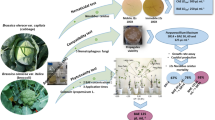
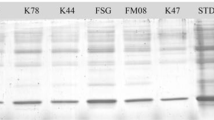
The plant-parasitic nematode Nacobbus aberrans is an endoparasite causing severe losses to a wide range of crops from North to South America. The use of native antagonistic fungi may be considered as a possible biological control alternative to reduce the damages caused by this species. Antagonistic effects of 66 potential nematophagous fungi against eggs (J1) and second-stage juveniles (J2) of N. aberrans, were evaluated in vitro on water agar. DGC test showed significant differences (p < 0.0001) in the efficacy of some fungal isolates tested, with parasitism levels for J1 and J2 of 0–95 and 1–78%, respectively. Five isolates of Purpureocillium lilacinum, Metarhizium robertsii and Plectosphaerella plurivora appeared as the most effective antagonists of N. aberrans, relying on hyphae and adhesive conidia in host infection processes.
Graphical Abstract









Similar content being viewed by others
References
Anthoine G, Mugniéry D (2005) Variability of the ITS rDNA and identification of Nacobbus aberrans (Thorne, 1935) Thorne and Allen, 1944 (Nematoda: Pratylenchidae) by rDNA amplification. Nematol 7:503–516
Atibalentja N, Noel GR, Ciancio A (2004) A simple method for the extraction, PCR-amplification, cloning, and sequencing of Pasteuria 16S rDNA from small numbers of endospores. J Nematol 36:100–105
Barra P, Rosso LC, Nesci A, Etcheverry MG (2013) Isolation and identification of entomopathogenic fungi and their evaluation against Tribolium confusum, Sitophilus zeamais, and Rhyzopertha dominica in stored maize. J Pest Sci 86:217–226
Dávila L, Hío JC (2005) Evauación biocontroladora de Arthrobotrys sp. y Paecilomyces sp. sobre Meloidogyne javanica in vitro y bajo condiciones de invernadero en crisantemo (Drendranthema grandiflora Anderson). Agron Colomb 23:223–229
Desaeger J, Rao MR (2000) Infection and damage potential of Meloidogyne javanica on Sesbania sesban in different soil types. Nematology 2:169–178
Di Rienzo JA, Guzmán AE, Casanoves F (2002) A multiple-comparisons method based on the distribution of the root node distance of a binary tree. J Agric Biol Environ Stat 7:129–142
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2012) “Infostat”. FCA, Universidad Nacional de Córdoba, Córdoba
Doucet ME (1989) The genus Nacobbus Thorne Allen, 1944 in Argentina. 1. Study of a population of N. aberrans (Thorne, 1935) Thorne & Allen, 1944 on Chenopodium album L. from Rio Cuarto, province of Cordoba. Rev Nématol 12:17–26
Doucet ME, Di Rienzo J (1991) El género Nacobbus Thorne & Allen, 1944 en Argentina. Caracterización morfológica y morfométrica de poblaciones de N. Aberrans (Thorne, 1935) Thorne & Allen, 1944. Nematropica 21:19–34
Doucet ME, Lax P (2005) El género Nacobbus Thorne & Allen, 1944 en la Argentina. 6. La especie N. Aberrans (Thorne, 1935) Thorne & Allen, 1944 (Nematoda: Tylenchida) y su relación con la agricultura. Acad Nac AyV LIX:5–45
EPPO (2011) PQR-EPPO database on quarantine pests. http://www.eppo.int
Fernández Lozano J (2012) La producción de hortalizas en Argentina. (Caracterización del sector y zonas de producción) Corporación del mercado central de Buenos Aires, 29. Informe técnico. http://www.central-servicios.com.ar/cmcba/ziptecnicas/la_produccion_de_hortalizas_en_argentina.pdf
Franco Navarro F, Vilchis Martinez K, Miranda Damian J (2008) New records of Pochonia chlamydosporia from mexico: isolation, root colonization and parasitism of Nacobbus aberrans eggs. Nematropica 39:133–142
Gortari MA, Hours RA (2016) Purpureocillium lilacinum LPSC # 876: producción de conidias en cultivos sobre sustratos sólidos y evaluación de su actividad sobre Nacobbus aberrans en plantas de tomate. Revista Fac Agron 115:2
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Humber RA (1998) Entomopathogenic fungal identification. Center for Agriculture and Health, Ithaca
Jaffee BA (1992) Population biology and biological control of nematodes. Can J Microbiol 38:359–364
Jaffee BA (2003) Correlations between most probable number and activity of nematode-trapping fungi. Phytopathology 93:1599–1605
Lopez-Llorca LV, Jansson HB (2007) Fungal parasites of invertebrates: multimodal biocontrol agents? In: Robson GD, van West P, Gadd GM (eds) Exploitation of fungi. Cambridge University Press, Cambridge, pp 310–335
Manzanilla-López RH, Costilla MA, Doucet ME, Franco J et al (2002) The genus Nacobbus Thorne & Allen, 1944 (Nematoda: Pratylenchidae): systematics, distribution, biology and management. Nematropica 32:149–228
Manzanilla-López RH, Esteves I, Finetti-Sialer MM, Hirsch PR, Ward E et al (2013) Pochonia chlamydosporia: advances and challenges to improve its performance as a biological control agent of sedentary endo-parasitic nematodes. J Nematol 45:1–7
Marro N, Lax P, Cabello M, Doucet ME, Becerra AG (2014) Use of the arbuscular mycorrhizal fungus Glomus intraradices as biological control agent of the nematode Nacobbus aberrans parasitizing tomato. Braz Arch Biol Technol 57:668–674
Méndoza de Gives P, Zavaleta Mejía E, Herrera Rodriguez D, Quiroz Romero H (1994) In vitro trapping capability of Arthrobotrys spp. on infective larvae of Haemonchus contortus and Nacobbus aberrans. J Helminthol 68:223–229
Nakajima ES, Ortega E (2015) Exploring the sustainable horticulture productions systems using the emergy assessment to restore the regional sustainability. J Clean Prod 96:531–538
Passone MA, Rosso LC, Ciancio A, Etcheverry MG (2010) Detection and quantification of Aspergillus section flavi spp. in stored peanuts by real-time PCR of nor-1 gene, and effects of storage conditions on aflatoxin production. Int J Food Microbiol 138:276–281
Peraza Padilla W, Orozco Aceves M, Esquivel Hernández A (2014) Evaluación in vitro de hongos nematófagos en zonas arroceras de Costa Rica contra el nematodo agallador Meloidogyne. Agron Costarric 38:19–32
Regaieg H, Ciancio A, Raouani NH, Rosso L (2011) Detection and biocontrol potential of Verticillium leptobactrum parasitizing Meloidogyne spp. World J Microbiol Biotechnol 27:1615–1623
Rosso LC (2009) Cloning, sequence, and expression analysis of a new MnSOD-encoding gene from the root-knot nematode Meloidogyne incognita. J Nematol 41:52–59
Ryss AY (2002) Express technique to prepare permanent collection slides of nematodes. Zoosyst Rossica 11:257–260
Samson RA, Evans HC, Latgé JP (1988) Atlas of entomopathogenic fungi. Springer, Berlin
Sosa AL, Passone MA, Rosso LC, Salusso F, Etcheverry M (2015) Aislamiento de nematodos y hongos con potencial actividad nematófaga en cultivos hortícolas del cinturón verde de la ciudad de Río Cuarto, Córdoba, Argentina. III Congreso Argentino de Microbiología Agrícola y Ambiental (CAMAYA), Buenos Aires
Stirling GR (2014) Biological control of plant parasitic nematodes: soil ecosystem management in sustainable agriculture, 2nd edn. CABI, Wallingford
Sturhan D, Schneider R (1980) Hirsutella heteroderae, a new nematode-parasitic fungus. Phytopathol Zeitschrift 99:105–115
Swe A, Li J, Zhang KQ, Pointing SB, Jeewon R, Hyde KD (2011) Nematode-trapping fungi. Curr Res Environ Appl Mycol 1:1–26
Talavera M, Itou K, Mizukubo T (2001) Reduction of nematode damage by root colonization with arbuscular mycorrhiza (Glomus spp.) in tomato Meloidogyne incognita (Tylenchida: Meloidogynidae) and carrot Pratylenchus penetrans (Tylenchida: Pratylenchidae) pathosystems. Appl Entomol Zool 36:387–392
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific Gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tyler J (1938) Egg output of the root-knot nematode. Proc Helminthol Soc Wash 5:49–54
Verdejo-Lucas S, Blanco M, Cortada L, Sorribas FJ (2013) Resistance of tomato rootstocks to Meloidogyne arenaria and Meloidogyne javanica under intermittent elevated soil temperatures above 28 °C. Crop Prot 46:57–62
White JH, Wise A, Main MJ, Green A et al (1998) Heterodimerization is required for the formation of a functional GABAb receptor. Nature 396:679–682
Yang J, Baoyu Tian LL, Ke QZ (2007) Extracellular enzymes and the pathogenesis of nematophagous fungi. Appl Microbiol Biotechnol 75:21–31
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7:203–214
Acknowledgements
This work was carried out by the grant from Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT), FONCYT-PICT/2016 No. 1005, 2017–2020 and Secretaría de Ciencia y Técnica, Universidad Nacional de Río Cuarto (SECYT-UNRC), PPI-2016 Res. 161, 2016–2018. The authors are grateful for the technical assistance of Dr. Alberto Troccoli, Istituto per la Protezione Sostenibile delle Piante, CNR, Bari, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Rights and permissions
About this article
Cite this article
Sosa, A.L., Rosso, L.C., Salusso, F.A. et al. Screening and identification of horticultural soil fungi for their evaluation against the plant parasitic nematode Nacobbus aberrans. World J Microbiol Biotechnol 34, 63 (2018). https://doi.org/10.1007/s11274-018-2441-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-018-2441-8