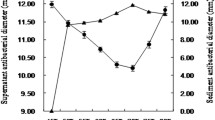
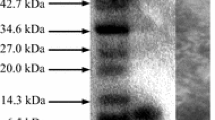
Abstract
Three bacteriocins from Lactobacillus plantarum KL-1 were successfully purified using ammonium sulfate precipitation, cation-exchange chromatography and reverse-phase HPLC. The bacteriocin peptides KL-1X, -1Y and -1Z had molecular masses of 3053.82, 3498.16 and 3533.16 Da, respectively. All three peptides were stable at pH 2–12 and 25 °C and at high temperatures of 80 and 100 °C for 30 min and 121 °C for 15 min. However, they differed in their susceptibility to proteolytic enzymes and their inhibition spectra. KL-1Y showed broad inhibitory activities against Gram-positive and Gram-negative bacteria, including Salmonella enterica serovar Enteritidis DMST 17368, Pseudomonas aeruginosa ATCC 15442, P. aeruginosa ATCC 9027, Escherichia coli O157:H7 and E. coli ATCC 8739. KL-1X and -1Z inhibited only Gram-positive bacteria. KL-1X, KL-1Y and KL-1Z exhibited synergistic activity. The successful amino acid sequencing of KL-1Y had a hydrophobicity of approximately 30 % and no cysteine residues suggested its novelty, and it was designated “plantaricin KL-1Y”. Plantaricin KL-1Y exhibited bactericidal activity against Bacillus cereus JCM 2152T. Compared to nisin, KL-1Y displayed broad inhibitory activities of 200, 800, 1600, 800, 400 and 400 AU/mL against the growth of Bacillus coagulans JCM 2257T, B. cereus JCM 2152T, Listeria innocua ATCC 33090T, Staphylococcus aureus TISTR 118, E. coli O157:H7 and E. coli ATCC 8739, respectively, whereas nisin had similar activities against only B. coagulans JCM 2257T and B. cereus JCM 2152T. Therefore, the novel plantaricin KL-1Y is a promising antimicrobial substance for food safety uses in the future.



Similar content being viewed by others
References
Anderssen EL, Diep DB, Nes IF, Eijsink VGH, Nissen-Meyer J (1998) Antagonistic activity of Lactobacillus plantarum C11: two new two-peptide bacteriocins, plantaricins EF and JK, and the induction factor plantaricin A. Appl Environ Microbiol 64:2269–2272
Atrih A, Rekhif N, Milliere JB, Lefebvre G (1993) Detection and characterization of a bacteriocin produced by Lactobacillus plantarum C19. Can J Microbiol 39:1173–1179. doi:10.1139/m93-178
Atrih A, Rekhif N, Moir A, Lebrihi A, Lefebvre G (2001) Mode of action, purification and amino acid sequence of plantaricin C19, an anti-Listeria bacteriocin produced by Lactobacillus plantarum C19. Int J Food Microbiol 68:93–104
Böhme K, Fernández-No IC, Barros-Velázquez J, Gallardo JM, Calo-Mata P, Cañas B (2010) Species differentiation of seafood spoilage and pathogenic Gram-negative bacteria by MALDI-TOF mass fingerprinting. J Proteome Res 9:3169–3183. doi:10.1021/pr100047q
Chen H, Hoover D (2003) Bacteriocins and their food applications. Compr Rev Food Sci Food Saf 2:82–100
Cintas LM, Casaus P, Holo H, Hernandez PE, Nes IF, HΣvarstein LS (1998) Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. J Bacteriol 180:1988–1994
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol 71:1–20. doi:10.1016/S0168-1605(01)00560-8
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788. doi:10.1038/nrmicro1273
Daeschel MA, Andersson RE, Fleming HP (1987) Microbial ecology of fermenting plant materials. FEMS Microbiol Lett 46:357–367. doi:10.1016/0378-1097(87)90119-4
Dallas DC, Underwood MA, Zivkovic AM, German JB (2012) Digestion of protein in premature and term infants. J Nutr Disord Ther 2:112. doi:10.4172/2161-0509.1000112
De Vuyst L, Vandamme E (1994) Antimicrobial potential of lactic acid bacteria. In: De Vuyst L, Vandamme E (eds) Bacteriocins of Lactic Acid Bacteria. Springer, US, pp 91–142. doi:10.1007/978-1-4615-2668-1_3
Delves-Broughton J, Blackburn P, Evans RJ, Hugenholtz J (1996) Applications of the bacteriocin, nisin. Antonie Van Leeuwenhoek 69:193–202. doi:10.1007/BF00399424
Dicks LMT, Mellett FD, Hoffman LC (2004) Use of bacteriocin-producing starter cultures of Lactobacillus plantarum and Lactobacillus curvatus in production of ostrich meat salami. Meat Sci 66:703–708. doi:10.1016/j.meatsci.2003.07.002
Diep BD, Havarstein LS, Nissen-Meyer J, Nes FI (1994) The gene encoding plantaricin A, a bacteriocin from Lactobacillus plantarum C11, is located on the same transcription unit as an agr- like regulatory system. Appl Environ Microbiol 60:160–166
Draper LA, Cotter PD, Hill C, Ross RP (2013) The two peptide lantibiotic lacticin 3147 acts synergistically with polymyxin to inhibit Gram negative bacteria. BMC Microbiol 13:212
Ehrmann MA, Remiger A, Eijsink VG, Vogel RF (2000) A gene cluster encoding plantaricin 1.25 and other bacteriocin-like peptides in Lactobacillus plantarum TMW1.25. Biochim Biophys Acta 1490:355–361
Elizaquível P, Chenoll E, Aznar R (2008) A TaqMan-based real-time PCR assay for the specific detection and quantification of Leuconostoc mesenteroides in meat products FEMS. Microbiol Lett 278:62–71. doi:10.1111/j.1574-6968.2007.00974.x
Enan G, El-Essawy AA, Uyttendaele M, Debevere J (1996) Antibacterial activity of Lactobacillus plantarum UG1 isolated from dry sausage: characterization, production and bactericidal action of plantaricin UG1. Int J Food Microbiol 30:189–215. doi:10.1016/0168-1605(96)00947-6
Ennahar S, Sashihara T, Sonomoto K, Ishizaki A (2000) Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol Rev 24:85–106. doi:10.1111/j.1574-6976.2000.tb00534.x
Ennahar S, Asou Y, Zendo T, Sonomoto K, Ishizaki A (2001) Biochemical and genetic evidence for production of enterocins A and B by Enterococcus faecium WHE 81. Int J Food Microbiol 70:291–301. doi:10.1016/S0168-1605(01)00565-7
Fricourt BV, Barefoot SF, Testin RF, Hayasaka SS (1994) Detection and activity of plantaricin F an antibacterial substance from Lactobacillus plantarum BF001 isolated from processed channel catfish. J Food Prot 57:698–702
Gong HS, Meng XC, Wang H (2010) Plantaricin MG active against Gram-negative bacteria produced by Lactobacillus plantarum KLDS1.0391 isolated from “Jiaoke”, a traditional fermented cream from China. Food Control 21:89–96. doi:10.1016/j.foodcont.2009.04.005
González B, Arca P, Mayo B, Suárez JE (1994) Detection, purification, and partial characterization of plantaricin C, a bacteriocin produced by a Lactobacillus plantarum strain of dairy origin. Appl Environ Microbiol 60:2158–2163
Hata T, Tanaka R, Ohmomo S (2010) Isolation and characterization of plantaricin ASM1: a new bacteriocin produced by Lactobacillus plantarum A-1. Int J Food Microbiol 137:94–99
Holo H, Jeknic Z, Daeschel M, Stevanovic S, Nes IF (2001) Plantaricin W from Lactobacillus plantarum belongs to a new family of two-peptide lantibiotics. Microbiology 147:643–651
Jiménez-Díaz R, Rios-Sánchez RM, Desmazeaud M, Ruiz-Barba JL, Piard J-C (1993) Plantaricins S and T, two new bacteriocins produced by Lactobacillus plantarum LPCO10 isolated from a green olive fermentation. Appl Environ Microbiol 59:1416–1424
Kelly WJ, Asmundson RV, Huang CM (1996) Characterization of plantaricin KW30, a bacteriocin produced by Lactobacillus plantarum. J Appl Bacteriol 81:657–662. doi:10.1111/j.1365-2672.1996.tb03561.x
Kimura H, Matsusaki H, Sashihara T, Sonomoto K, Ishizaki A (1998) Purification and partial identification of bacteriocin ISK-1, a new lantibiotic produced by Pediococcus sp. ISK-1. Biosci Biotechnol Biochem 62:2341–2345. doi:10.1271/bbb.62.2341
Leal-Sánchez MV, Ruiz-Barba JL, Sánchez AH, Rejano L, Jiménez-Díaz R, Garrido A (2003) Fermentation profile and optimization of green olive fermentation using Lactobacillus plantarum LPCO10 as a starter culture. Food Microbiol 20:421–430. doi:10.1016/S0740-0020(02)00147-8
Maldonado A, Ruiz-Barba JL, Jimenez-Diaz R (2003) Purification and genetic characterization of plantaricin NC8, a novel coculture-inducible two-peptide bacteriocin from Lactobacillus plantarum NC8. Appl Environ Microbiol 69:383–389
Masuda Y, Zendo T, Sawa N, Perez RH, Nakayama J, Sonomoto K (2012) Characterization and identification of weissellicin Y and weissellicin M, novel bacteriocins produced by Weissella hellenica QU 13. J Appl Microbiol 112:99–108. doi:10.1111/j.1365-2672.2011.05180.x
Müller DM, Carrasco MS, Simonetta AC, Beltramini LM, Tonarelli GG (2007) A synthetic analog of plantaricin 149 inhibiting food-borne pathogenic bacteria:evidence for α-helical conformation involved in bacteria–membrane interaction. J Pept Sci 13:171–178. doi:10.1002/psc.828
Nissen-Meyer J, Larsen AG, Sletten K, Daeschel M, Nes IF (1993) Purification and characterization of plantaricin A, a Lactobacillus plantarum bacteriocin whose activity depends on the action of two peptides. J Gen Microbiol 139:1973–1978. doi:10.1099/00221287-139-9-1973
Omar NB, Abriouel H, Lucas R, Martínez-Cañamero M, Guyot J-P, Gálvez A (2006) Isolation of bacteriocinogenic Lactobacillus plantarum strains from ben saalga, a traditional fermented gruel from Burkina Faso. Int J Food Microbiol 112:44–50. doi:10.1016/j.ijfoodmicro.2006.06.014
Parente E, Brienza C, Moles M, Ricciardi A (1995) A comparison of methods for the measurement of bacteriocin activity. J Microbiol Methods 22:95–108. doi:10.1016/0167-7012(94)00068-I
Perez RH, Zendo T, Sonomoto K (2014) Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb Cell Fact 13:S3
Rekhif N, Atrih A, Lefebvre G (1994) Characterization and partial purification of plantaricin LC74, a bacteriocin produced by Lactobacillus plantarum LC74. Biotechnol Lett 16:771–776. doi:10.1007/BF00133952
Rekhif N, Atrih A, Lefebvrexy G (1995) Activity of plantaricin SA6, a bacteriocin produced by Lactobacillus plantarum SA6 isolated from fermented sausage. J Appl Bacteriol 78:349–358. doi:10.1111/j.1365-2672.1995.tb03417.x
Sashihara T, Kimura H, Higuchi T, Adachi A, Matsusaki H, Sonomoto K, Ishizaki A (2000) A novel lantibiotic, nukacin ISK-1, of Staphylococcus warneri ISK-1: cloning of the structural gene and identification of the structure. Biosci Biotechnol Biochem 64:2420–2428
Sawa N, Zendo T, Kiyofuji J, Fujita K, Himeno K, Nakayama J, Sonomoto K (2009) Identification and characterization of lactocyclicin Q, a novel cyclic bacteriocin produced by Lactococcus sp. strain QU 12. Appl Environ Microbiol 75:1552–1558. doi:10.1128/aem.02299-08
Sawa N, Okamura K, Zendo T, Himeno K, Nakayama J, Sonomoto K (2010) Identification and characterization of novel multiple bacteriocins produced by Leuconostoc pseudomesenteroides QU 15. J Appl Microbiol 109:282–291. doi:10.1111/j.1365-2672.2009.04653.x
Sawa N, Koga S, Okamura K, Ishibashi N, Zendo T, Sonomoto K (2013) Identification and characterization of novel multiple bacteriocins produced by Lactobacillus sakei D98. J Appl Microbiol 115:61–69. doi:10.1111/jam.12226
Schillinger U, Lücke FK (1989) Antibacterial activity of Lactobacillus sake isolated from meat. Appl Environ Microbiol 55:1901–1906
Schirmer BC, Heir E, Langsrud S (2009) Characterization of the bacterial spoilage flora in marinated pork products. J Appl Microbiol 106:2106–2116. doi:10.1111/j.1365-2672.2009.04183.x
Tagg J, McGiven A (1971) Assay system for bacteriocins. Appl Microbiol 21:943
Todorov SD, Vaz-Velho M, Gibbs P (2004) Comparison of two methods for purification of plantaricin ST31, a bacteriocin produced by Lactobacillus plantarum ST31. Braz J Microbiol 35:157–160
Todorov SD, Nyati H, Meincken M, Dicks LMT (2007) Partial characterization of bacteriocin AMA-K, produced by Lactobacillus plantarum AMA-K isolated from naturally fermented milk from Zimbabwe. Food Control 18:656–664. doi:10.1016/j.foodcont.2006.03.003
van Reenen CA, Dicks LM, Chikindas ML (1998) Isolation, purification and partial characterization of plantaricin 423, a bacteriocin produced by Lactobacillus plantarum. J Appl Microbiol 84:1131–1137
Wilaipun P, Zendo T, Sangjindavong M, Nitisinprasert S, Leelawatcharamas V, Nakayama J, Sonomoto K (2004) The two-synergistic peptide bacteriocin produced by Enterococcus faecium NKR-5-3 isolated from Thai fermented fish (Pla-ra). Sci Asia 30:115–122
Zendo T, Koga S, Shigeri Y, Nakayama J, Sonomoto K (2006) Lactococcin Q, a novel two-peptide bacteriocin produced by Lactococcus lactis QU 4. Appl Environ Microbiol 72:3383–3389. doi:10.1128/aem.72.5.3383-3389.2006
Acknowledgments
We express our gratitude to The Royal Golden Jubilee Ph.D. program for a Ph.D. scholarship. Our thanks also go to S&P Syndicate Public Company Limited and the Laboratory of Microbial Technology, Division of Microbial Science and Technology, Department of Bioscience and Biotechnology, Faculty of Agriculture, Graduate School, Kyushu University, Fukuoka, Japan.
Conflict of Interests
We have no conflict of interest.
Funding
This study was funded by The Royal Golden Jubilee Ph.D. program, Thailand.
Ethical approval
Not required.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rumjuankiat, K., Perez, R.H., Pilasombut, K. et al. Purification and characterization of a novel plantaricin, KL-1Y, from Lactobacillus plantarum KL-1. World J Microbiol Biotechnol 31, 983–994 (2015). https://doi.org/10.1007/s11274-015-1851-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1851-0