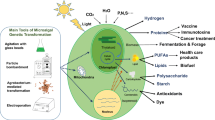
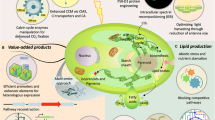
Abstract
Microalgae have been used in food, cosmetic, and biofuel industries as a natural source of lipids, vitamins, pigments and antioxidants for a long time. Green microalgae, as potent photobioreactors, can be considered as an economical expression system to produce recombinant therapeutical proteins at large-scale due to low cost of production and scaling-up capitalization owning to the inexpensive medium requirement, fast growth rate, and the ease of manipulation. These microalgae possess all benefit eukaryotic expression systems including the ability of post-translational modifications required for proper folding and stability of active proteins. Among the many items regarded as recombinant protein production, this review compares the different expression systems with green microalgae like Dunaliella by viewing the nuclear/chloroplast transformation challenges/benefits, related selection markers/reporter genes, and crucial factors/strategies affecting the increase of foreign protein expression in microalgae transformants. Some important factors were discussed regarding the increase of protein yielding in microalgae transformants including: transformation-associated genotypic modifications, endogenous regulatory factors, promoters, codon optimization, enhancer elements, and milking of recombinant protein.


Similar content being viewed by others
References
Abranches R, Shultz RW, Thompson WF, Allen GC (2005) Matrix attachment regions and regulated transcription increase and stabilize transgene expression. Plant Biotechnol J 3:535–543
Allen GC, Spiker S, Thompson WF (2005) Transgene integration: use of matrix attachment regions. Methods Mol Biol 286:313–326
Ascenzi R, Ulker B, Todd JJ et al (2003) Analysis of trans-silencing interactions using transcriptional silencers of varying strength and targets with and without flanking nuclear matrix attachment regions. Transgenic Res 12:305–318
Barzegari A, Hejazi MA, Hosseinzadeh N, Eslami S, Mehdizadeh AE, Hejazi MS (2010) Dunaliella as an attractive candidate for molecular farming. Mol Biol Rep 37:3427–3430
Ben-Amotz A, Avron M (1983) On the factors which determine massive beta-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol 72:593–597
Ben-Amotz A, Edelstein S, Avron M (1986) Use of the beta-carotene rich alga Dunaliella bardawil as a source of retinol. Br Poult Sci 27:613–619
Ben-Amotz A, Lers A, Avron M (1988) Stereoisomers of beta-carotene and phytoene in the alga Dunaliella bardawil. Plant Physiol 86:1286–1291
Ben-Amotz A, Shaish A, Avron M (1989) Mode of action of the massively accumulated beta-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiol 91:1040–1043
Berthold P, Schmitt R, Mages W (2002) An engineered Streptomyces hygroscopicus aph 7″ gene mediates dominant resistance against hygromycin B in Chlamydomonas reinhardtii. Protist 153:401–412
Bock R (2001) Transgenic plastids in basic research and plant biotechnology. J Mol Biol 312:425–438
Boehm R (2007) Bioproduction of therapeutic proteins in the 21st century and the role of plants and plant cells as production platforms. Ann NY Acad Sci 1102:121–134
Brown LE, Sprecher SL, Keller LR (1991) Introduction of exogenous DNA into Chlamydomonas reinhardtii by electroporation. Mol Cell Biol 11:2328–2332
Cereghino JL, Cregg JM (2000) Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev 24:45–66
Chebolu S, Daniell H (2007) Stable expression of Gal/GalNAc lectin of Entamoeba histolytica in transgenic chloroplasts and immunogenicity in mice towards vaccine development for amoebiasis. Plant Biotechnol J 5:230–239
Cheirsilp B, Suwannarat W, Niyomdecha R (2011) Mixed culture of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for lipid production from industrial wastes and its use as biodiesel feedstock. N Biotechnol 28:362–368
Conrad U, Fiedler U (1994) Expression of engineered antibodies in plant cells. Plant Mol Biol 26:1023–1030
Conrad U, Fiedler U (1998) Compartment-specific accumulation of recombinant immunoglobulins in plant cells: an essential tool for antibody production and immunomodulation of physiological functions and pathogen activity. Plant Mol Biol 38:101–109
Conrad U, Manteuffel R (2001) Immunomodulation of phytohormones and functional proteins in plant cells. Trends Plant Sci 6:399–402
Coragliotti AT, Beligni MV, Franklin SE, Mayfield SP (2011) Molecular factors affecting the accumulation of recombinant proteins in the Chlamydomonas reinhardtii chloroplast. Mol Biotechnol 48:60–75
Courchesne NM, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141:31–41
Daniell H (2006) Production of biopharmaceuticals and vaccines in plants via the chloroplast genome. Biotechnol J 1:1071–1079
Daniell H, Streatfield SJ, Wycoff K (2001) Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci 6:219–226
Daniell H, Ruiz G, Denes B, Sandberg L, Langridge W (2009) Optimization of codon composition and regulatory elements for expression of human insulin like growth factor-1 in transgenic chloroplasts and evaluation of structural identity and function. BMC Biotechnol 9:33
Deruere J, Romer S, d’Harlingue A, Backhaus RA, Kuntz M, Camara B (1994) Fibril assembly and carotenoid overaccumulation in chromoplasts: a model for supramolecular lipoprotein structures. Plant Cell 6:119–133
Doran MR, Markway BD, Croll TI, Sara S, Munro TP, Cooper-White JJ (2009) Controlled presentation of recombinant proteins via a zinc-binding peptide-linker in two and three dimensional formats. Biomaterials 30:6614–6620
Dumetz AC, Snellinger-O’brien AM, Kaler EW, Lenhoff AM (2007) Patterns of protein protein interactions in salt solutions and implications for protein crystallization. Protein Sci 16:1867–1877
Eichler-Stahlberg A, Weisheit W, Ruecker O, Heitzer M (2009) Strategies to facilitate transgene expression in Chlamydomonas reinhardtii. Planta 229:873–883
Faye L, Daniell H (2006) Novel pathways for glycoprotein import into chloroplasts. Plant Biotechnol J 4:275–279
Feng S, Xue L, Liu H, Lu P (2009) Improvement of efficiency of genetic transformation for Dunaliella salina by glass beads method. Mol Biol Rep 36:1433–1439
Franklin SE, Mayfield SP (2005) Recent developments in the production of human therapeutic proteins in eukaryotic algae. Expert Opin Biol Ther 5:225–235
Gantar M, Berry JP, Thomas S, Wang M, Perez R, Rein KS (2008) Allelopathic activity among cyanobacteria and microalgae isolated from Florida freshwater habitats. FEMS Microbiol Ecol 64:55–64
Geng D, Wang Y, Wang P, Li W, Sun Y (2003) Stable expression of hepatitis B surface antigen gene in Dunaliella salina (Chlorophyta). J Appl Phycol 15:451–456
Geng T, Zhan Y, Wang HY, Witting SR, Cornetta KG, Lu C (2010) Flow-through electroporation based on constant voltage for large-volume transfection of cells. J Control Release 144:91–100
Geng T, Zhan Y, Wang J, Lu C (2011) Transfection of cells using flow-through electroporation based on constant voltage. Nat Protoc 6:1192–1208
Gong Y, Hu H, Gao Y, Xu X, Gao H (2011) Microalgae as platforms for production of recombinant proteins and valuable compounds: progress and prospects. J Ind Microbiol Biotechnol 38:1879–1890
Greenwell HC, Laurens LM, Shields RJ, Lovitt RW, Flynn KJ (2010) Placing microalgae on the biofuels priority list: a review of the technological challenges. J R Soc Interface 7:703–726
Heid HW, Keenan TW (2005) Intracellular origin and secretion of milk fat globules. Eur J Cell Biol 84:245–258
Heitzer M, Eckert A, Fuhrmann M, Griesbeck C (2007) Influence of codon bias on the expression of foreign genes in microalgae. Adv Exp Med Biol 616:46–53
Hejazi MA, Kleinegris D, Wijffels RH (2004) Mechanism of extraction of beta-carotene from microalga Dunaliella salina in two-phase bioreactors. Biotechnol Bioeng 88:593–600
Huang JJ, Cheung PC (2011) Enhancement of polyunsaturated fatty acids and total carotenoid production in microalgae by ultraviolet band A (UVA, 365 nm) radiation. J Agric Food Chem 59:4629–4636
Ikonomou L, Schneider YJ, Agathos SN (2003) Insect cell culture for industrial production of recombinant proteins. Appl Microbiol Biotechnol 62:1–20
James VA, Avart C, Worland B, Snape JW, Vain P (2002) The relationship between homozygous and hemizygous transgene expression levels over generations in populations of transgenic rice plants. Theor Appl Genet 104:553–561
Jarvis P (2003) Intracellular signalling: the language of the chloroplast. Curr Biol 13:R314–R316
Jarvis P (2008) Targeting of nucleus-encoded proteins to chloroplasts in plants. New Phytol 179:257–285
Jarvis P, Robinson C (2004) Mechanisms of protein import and routing in chloroplasts. Curr Biol 14:R1064–R1077
Jia Y, Li S, Allen G, Feng S, Xue L (2012) A novel glyceraldehyde-3-phosphate dehydrogenase (GAPDH) promoter for expressing transgenes in the halotolerant alga Dunaliella salina. Curr Microbiol 64:506–513
Jiang GZ, Lu YM, Niu XL, Xue LX (2005) The actin gene promoter-driven bar as a dominant selectable marker for nuclear transformation of Dunaliella salina. Yi Chuan Xue Bao 32:424–433
Jimenez C, Pick U (1993) Differential reactivity of [beta]-carotene isomers from Dunaliella bardawil toward oxygen radicals. Plant Physiol 101:385–390
Jones CS, Luong T, Hannon M et al (2012) Heterologous expression of the C-terminal antigenic domain of the malaria vaccine candidate Pfs48/45 in the green algae Chlamydomonas reinhardtii. Appl Microbiol Biotechnol 97:1987–1995
Katz A, Jimenez C, Pick U (1995) Isolation and characterization of a protein associated with carotene globules in the alga Dunaliella bardawil. Plant Physiol 108:1657–1664
Katz A, Waridel P, Shevchenko A, Pick U (2007) Salt-induced changes in the plasma membrane proteome of the halotolerant alga Dunaliella salina as revealed by blue native gel electrophoresis and nano-LC-MS/MS analysis. Mol Cell Proteomics 6:1459–1472
Kim JM, Kim JS, Park DH et al (2004) Improved recombinant gene expression in CHO cells using matrix attachment regions. J Biotechnol 107:95–105
Kleinegris DM, Janssen M, Brandenburg WA, Wijffels RH (2010) The selectivity of milking of Dunaliella salina. Mar Biotechnol (NY) 12:14–23
Lam MK, Lee KT (2011) Microalgae biofuels: a critical review of issues, problems and the way forward. Biotechnol Adv 30:673–690
Lao YM, Xiao L, Ye ZW, Jiang JG, Zhou SS (2011) In silico analysis of phytoene synthase and its promoter reveals hints for regulation mechanisms of carotenogenesis in Duanliella bardawil. Bioinformatics 27:2201–2208
Lee SM, Kang K, Chung H et al (2006) Plastid transformation in the monocotyledonous cereal crop, rice (Oryza sativa) and transmission of transgenes to their progeny. Mol Cells 21:401–410
Leon-Banares R, Gonzalez-Ballester D, Galvan A, Fernandez E (2004) Transgenic microalgae as green cell-factories. Trends Biotechnol 22:45–52
Li SS, Tsai HJ (2009) Transgenic microalgae as a non-antibiotic bactericide producer to defend against bacterial pathogen infection in the fish digestive tract. Fish Shellfish Immunol 26:316–325
Li J, Xue L, Yan H et al (2007) The nitrate reductase gene-switch: a system for regulated expression in transformed cells of Dunaliella salina. Gene 403:132–142
Li Q, Du W, Liu D (2008) Perspectives of microbial oils for biodiesel production. Appl Microbiol Biotechnol 80:749–756
Li J, Lu Y, Xue L, Xie H (2010) A structurally novel salt-regulated promoter of duplicated carbonic anhydrase gene 1 from Dunaliella salina. Mol Biol Rep 37:1143–1154
Long ZF, Wang SY, Nelson N (1989) Cloning and nucleotide sequence analysis of genes coding for the major chlorophyll-binding protein of the moss Physcomitrella patens and the halotolerant alga Dunaliella salina. Gene 76:299–312
Lutz KA, Maliga P (2007) Construction of marker-free transplastomic plants. Curr Opin Biotechnol 18:107–114
Lutz KA, Svab Z, Maliga P (2006) Construction of marker-free transplastomic tobacco using the Cre-loxP site-specific recombination system. Nat Protoc 1:900–910
Mankin SL, Allen GC, Phelan T, Spiker S, Thompson WF (2003) Elevation of transgene expression level by flanking matrix attachment regions (MAR) is promoter dependent: a study of the interactions of six promoters with the RB7 3’ MAR. Transgenic Res 12:3–12
Manuell AL, Beligni MV, Elder JH et al (2007) Robust expression of a bioactive mammalian protein in Chlamydomonas chloroplast. Plant Biotechnol J 5:402–412
Mayfield SP, Franklin SE (2005) Expression of human antibodies in eukaryotic micro-algae. Vaccine 23:1828–1832
Mayfield SP, Franklin SE, Lerner RA (2003) Expression and assembly of a fully active antibody in algae. Proc Natl Acad Sci USA 100:438–442
Michalowski SM, Allen GC, Hall GE Jr, Thompson WF, Spiker S (1999) Characterization of randomly-obtained matrix attachment regions (MARs) from higher plants. Biochemistry 38:12795–12804
Michelet L, Lefebvre-Legendre L, Burr SE, Rochaix JD, Goldschmidt-Clermont M (2011) Enhanced chloroplast transgene expression in a nuclear mutant of Chlamydomonas. Plant Biotechnol J 9:565–574
Minai L, Wostrikoff K, Wollman FA, Choquet Y (2006) Chloroplast biogenesis of photosystem II cores involves a series of assembly-controlled steps that regulate translation. Plant Cell 18:159–175
Mishra A, Mandoli A, Jha B (2008) Physiological characterization and stress-induced metabolic responses of Dunaliella salina isolated from salt pan. J Ind Microbiol Biotechnol 35:1093–1101
Muto M, Henry RE, Mayfield SP (2009) Accumulation and processing of a recombinant protein designed as a cleavable fusion to the endogenous Rubisco LSU protein in Chlamydomonas chloroplast. BMC Biotechnol 9:26
Neupert J, Karcher D, Bock R (2009) Generation of Chlamydomonas strains that efficiently express nuclear transgenes. Plant J 57:1140–1150
Neupert J, Shao N, Lu Y, Bock R (2012) Genetic transformation of the model green alga Chlamydomonas reinhardtii. Methods Mol Biol 847:35–47
Newman SM, Boynton JE, Gillham NW, Randolph-Anderson BL, Johnson AM, Harris EH (1990) Transformation of chloroplast ribosomal RNA genes in Chlamydomonas: molecular and genetic characterization of integration events. Genetics 126:875–888
Newman SM, Harris EH, Johnson AM, Boynton JE, Gillham NW (1992) Nonrandom distribution of chloroplast recombination events in Chlamydomonas reinhardtii: evidence for a hotspot and an adjacent cold region. Genetics 132:413–429
Noll F, May C, Kindl H (2000) Phospholipid monolayer of plant lipid bodies attacked by phospholipase A2 shows 80 nm holes analyzed by atomic force microscopy. Biophys Chem 86:29–35
Oren A (2005) A hundred years of Dunaliella research: 1905–2005. Saline Syst 1:2
Peach C, Velten J (1991) Transgene expression variability (position effect) of CAT and GUS reporter genes driven by linked divergent T-DNA promoters. Plant Mol Biol 17:49–60
Perreault F, Bogdan N, Morin M, Claverie J, Popovic R (2011) Interaction of gold nanoglycodendrimers with algal cells (Chlamydomonas reinhardtii) and their effect on physiological processes. Nanotoxicology 6:109–120
Pighin JA, Zheng H, Balakshin LJ et al (2004) Plant cuticular lipid export requires an ABC transporter. Science 306:702–704
Polle JE, Struwe L, Jin E (2008) Identification and characterization of a new strain of the unicellular green alga Dunaliella salina (Teod.) from Korea. J Microbiol Biotechnol 18:821–827
Potvin G, Zhang Z (2010) Strategies for high-level recombinant protein expression in transgenic microalgae: a review. Biotechnol Adv 28:910–918
Primrose SB, Ehrlich SD (1981) Isolation of plasmid deletion Mutants and study of their instability. Plasmid 6:193–201
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9:486–501
Radakovits R, Eduafo PM, Posewitz MC (2011) Genetic engineering of fatty acid chain length in Phaeodactylum tricornutum. Metab Eng 13:89–95
Rajamani S, Siripornadulsil S, Falcao V, Torres M, Colepicolo P, Sayre R (2007) Phycoremediation of heavy metals using transgenic microalgae. Adv Exp Med Biol 616:99–109
Rasala BA, Mayfield SP (2011) The microalga Chlamydomonas reinhardtii as a platform for the production of human protein therapeutics. Bioeng Bugs 2:50–54
Rasala BA, Muto M, Lee PA et al (2010) Production of therapeutic proteins in algae, analysis of expression of seven human proteins in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol J 8:719–733
Rasala BA, Muto M, Sullivan J, Mayfield SP (2011) Improved heterologous protein expression in the chloroplast of Chlamydomonas reinhardtii through promoter and 5′ untranslated region optimization. Plant Biotechnol J 9:674–683
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ (2008) A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19:430–436
Sadka A, Himmelhoch S, Zamir A (1991) A 150 kilodalton cell surface protein is induced by salt in the halotolerant green alga Dunaliella salina. Plant Physiol 95:822–831
Sanford JC, Smith FD, Russell JA (1993) Optimizing the biolistic process for different biological applications. Methods Enzymol 217:483–509
Schroda M, Blocker D, Beck CF (2000) The HSP70A promoter as a tool for the improved expression of transgenes in Chlamydomonas. Plant J 21:121–131
Schroda M, Beck CF, Vallon O (2002) Sequence elements within an HSP70 promoter counteract transcriptional transgene silencing in Chlamydomonas. Plant J 31:445–455
Siripornadulsil S, Traina S, Verma DP, Sayre RT (2002) Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 14:2837–2847
Siripornadulsil S, Dabrowski K, Sayre R (2007) Microalgal vaccines. Adv Exp Med Biol 616:122–128
Smirra I, Halevy AH, Vainstein A (1993) Isolation and characterization of a chromoplast-specific carotenoid-associated protein from Cucumis sativus corollas. Plant Physiol 102:491–496
Smith FD, Harpending PR, Sanford JC (1992) Biolistic transformation of prokaryotes: factors that affect biolistic transformation of very small cells. J Gen Microbiol 138:239–248
Smith DR, Lee RW, Cushman JC, Magnuson JK, Tran D, Polle JE (2010) The Dunaliella salina organelle genomes: large sequences, inflated with intronic and intergenic DNA. BMC Plant Biol 10:83
Sun Y, Yang Z, Gao X, Li Q, Zhang Q, Xu Z (2005) Expression of foreign genes in Dunaliella by electroporation. Mol Biotechnol 30:185–192
Sun G, Zhang X, Sui Z, Mao Y (2008) Inhibition of pds gene expression via the RNA interference approach in Dunaliella salina (Chlorophyta). Mar Biotechnol (NY) 10:219–226
Surzycki R, Greenham K, Kitayama K et al (2009) Factors effecting expression of vaccines in microalgae. Biologicals 37:133–138
Tan C, Qin S, Zhang Q, Jiang P, Zhao F (2005) Establishment of a micro-particle bombardment transformation system for Dunaliella salina. J Microbiol 43:361–365
Thanh T, Chi VT, Abdullah MP, Omar H, Noroozi M, Napis S (2011) Cloning and characterization of ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit (RbcS) cDNA from green microalga Ankistrodesmus convolutus. Mol Biol Rep 38:5297–5305
Tran M, Zhou B, Pettersson PL, Gonzalez MJ, Mayfield SP (2009) Synthesis and assembly of a full-length human monoclonal antibody in algal chloroplasts. Biotechnol Bioeng 104:663–673
Van der Vyver C, Schneidereit J, Driscoll S, Turner J, Kunert K, Foyer CH (2003) Oryzacystatin I expression in transformed tobacco produces a conditional growth phenotype and enhances chilling tolerance. Plant Biotechnol J 1:101–112
van Wijk KJ (2004) Plastid proteomics. Plant Physiol Biochem 42:963–977
Van LW, Ruttink T, Borst-Vrenssen AW, van der Plas LH, van der Krol AR (2001) Characterization of position-induced spatial and temporal regulation of transgene promoter activity in plants. J Exp Bot 52:949–959
Walker TL, Becker DK, Collet C (2005) Characterisation of the Dunaliella tertiolecta RbcS genes and their promoter activity in Chlamydomonas reinhardtii. Plant Cell Rep 23:727–735
Wang TY, Hou WH, Chai YR, Ji X, Wang JM, Xue LX (2005a) Nuclear matrices and matrix attachment regions from green alga: Dunaliella salina. Yi Chuan Xue Bao 32:1312–1318
Wang TY, Hou WH, Yuan BM et al (2005b) Construction of randomly matrix attachment regions library from the green alga: Dunaliella salina. Shi Yan Sheng Wu Xue Bao 38:23–28
Wang T, Xue L, Hou W, Yang B, Chai Y, Wang Y (2007) Increased expression of transgene in stably transformed cells of Dunaliella salina by matrix attachment regions. Appl Microbiol Biotechnol 76:651–657
Williams PJ (2007) Biofuel: microalgae cut the social and ecological costs. Nature 450:478
Williams PR, Inman D, Aden A, Heath GA (2009) Environmental and sustainability factors associated with next-generation biofuels in the U.S.: what do we really know? Environ Sci Technol 43:4763–4775
Wirth S, Calamante G, Mentaberry A, Bussmann L, Lattanzi M, Barañao L, Bravo-Almonacid F (2004) Expression of active human epidermal growth factor (hEGF) in tobacco plants by integrative and non-integrative systems. Mol Breed 13:23–35
Wostrikoff K, Choquet Y, Wollman FA, Girard-Bascou J (2001) TCA1, a single nuclear-encoded translational activator specific for petA mRNA in Chlamydomonas reinhardtii chloroplast. Genetics 159:119–132
Wostrikoff K, Girard-Bascou J, Wollman FA, Choquet Y (2004) Biogenesis of PSI involves a cascade of translational autoregulation in the chloroplast of Chlamydomonas. EMBO J 23:2696–2705
Xia X (2007) An improved implementation of codon adaptation index. Evol Bioinform Online 3:53–58
Zhang X, Stettler M, Reif O et al (2008) Shaken helical track bioreactors: providing oxygen to high-density cultures of mammalian cells at volumes up to 1000 L by surface aeration with air. N Biotechnol 25:68–75
Zhang H, Wang W, Quan C, Fan S (2010) Engineering considerations for process development in mammalian cell cultivation. Curr Pharm Biotechnol 11:103–112
Acknowledgments
The financial support of the Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences is gratefully acknowledged.
Conflict of interest
There is no conflict of interests to be declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akbari, F., Eskandani, M. & Khosroushahi, A.Y. The potential of transgenic green microalgae; a robust photobioreactor to produce recombinant therapeutic proteins. World J Microbiol Biotechnol 30, 2783–2796 (2014). https://doi.org/10.1007/s11274-014-1714-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-014-1714-0