Abstract
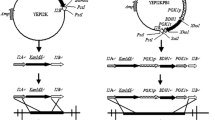
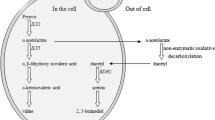
The flavor stability during storage is very important to the freshness and shelf life of beer. However, beer fermented with a yeast strain which is prone to autolyze will significantly affect the flavor of product. In this study, the gene encoding β-1,3-glucan synthetase catalytic subunit (fks1) of the lager yeast was destroyed via self-clone strategy. β-1,3-glucan is the principle cell wall component, so fks1 disruption caused a decrease in β-1,3-glucan level and increase in chitin level in cell wall, resulting in the increased cell wall thickness. Comparing with wild-type strain, the mutant strain had 39.9 and 63.41 % less leakage of octanoic acid and decanoic acid which would significantly affect the flavor of beer during storage. Moreover, the results of European Brewery Convention tube fermentation test showed that the genetic manipulation to the industrial brewing yeast helped with the anti-staling ability, rather than affecting the fermentation ability. The thiobarbituric acid value reduced by 65.59 %, and the resistant staling value increased by 26.56 %. Moreover, the anti-staling index of the beer fermented with mutant strain increased by 2.64-fold than that from wild-type strain respectively. China has the most production and consumption of beer around the world, so the quality of beer has a significant impact on Chinese beer industry. The result of this study could help with the improvement of the quality of beer in China as well as around the world.



Similar content being viewed by others
References
Burke D, Dawson D, Stearns T (2000) Methods in yeast genetics: a cold spring harbor laboratory course manual, 2000th edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Cabib E, Shaw JA, Mol PC, Bower B, Choi WJ (1996) Biochemistry and molecular biology, vol 3. Springer, NY
Cameron-Clarke A, Hulse GA, Clifton L, Cantrell IC (2003) The use of adenylate kinase measurement to determine causes of lysis in lager yeast. J Am Soc Brew Chem 61(3):152–156
Ernest CH, Jamieson AM, Gheluwe GV (1980) The release of fatty acids as a consequence of yeast autolysis. J Am Soc Brew Chem 38(1):13
François JM (2007) A simple method for quantitative determination of polysaccharides in fungal cell walls. Nat Protoc 1(6):2995–3000
Fu ZH, Li Q, Gu G (2004) Forecast of beer staling-discussion on the the method of TBA. Food Ferment Ind 30(3):97–101 (In Chinese)
Garcia-Rodriguez L, Trilla J, Castro C, Valdivieso M, Duran A, Roncero C (2000) Characterization of the chitin biosynthesis process as a compensatory mechanism in the fks1 mutant of Saccharomyces cerevisiae. FEBS Lett 478(1–2):84
Gibson BR, Lawrence SJ, Leclaire JPR, Powell CD, Smart KA (2007) Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol Rev 31(5):535–569. doi:10.1111/j.1574-6976.2007.00076.x
Gu G (1996) Brewing science and technology. China Light Industry Press, Beijing (In Chinese)
Hao QJ, Li Q, Gu G (2005) Yeast autolysis and its effect on beer quality. Beer Technol 1:33–34 (In Chinese)
Hernawan T, Fleet G (1995) Chemical and cytological changes during the autolysis of yeasts. J Ind Microbiol Biot 14(6):440–450
Inouhe M, Sugo E, Tohoyama H, Joho M, Nevins DJ (1997) Cell wall metabolism and autolytic activities of the yeast Saccharomyces exiguus. Int J Biol Macromol 21(1–2):11–14
Jollès P, Muzzarelli RAA (1999) Chitin and chitinases, vol 87. Berlin, Springer
Kapteyn JC, Van Den Ende H, Klis FM (1999) The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim Biophys Acta 1426(2):373–383. doi:10.1016/S0304-4165(98)00137-8
Li Q (1998) Research on the beer flavor and the flavor stability. Jiangnan University, Wuxi (In Chinese)
Liu CF, Zheng FY, Li YX, Li Q, Gu G (2008) Determination of free fatty acids in beer by multilevel solvent extraction-gas chromatography. Chin J Anal Lab 27(2):26–29 (In Chinese)
Mahajan BVC, Singh K, Dhillon W (2010) Effect of 1-methylcyclopropene (1-MCP) on storage life and quality of pear fruits. J Food Sci Tech 47(3):351–354
Marangon M, Van Sluyter SC, Haynes PA, Waters EJ (2009) Grape and wine proteins: their fractionation by hydrophobic interaction chromatography and identification by chromatographic and proteomic analysis. J Agric Food Chem 57(10):4415–4425. doi:10.1021/jf9000742
Martınez-Rodriguez A, Carrascosa A, Polo M (2001) Release of nitrogen compounds to the extracellular medium by three strains of Saccharomyces cerevisiae during induced autolysis in a model wine system. Int J Food Microbiol 68(1):155–160
Min Y (2005) The estimation of endogenous antioxidative activity of beer by analysis of DPPH radical scavenging capacity. Sci Technol Food Ind 8:024
Palomero F, Morata A, Benito S, González M, Suárez-Lepe J (2007) Conventional and enzyme-assisted autolysis during ageing over lees in red wines: influence on the release of polysaccharides from yeast cell walls and on wine monomeric anthocyanin content. Food Chem 105(2):838–846
Parsons R, Cope R (1983) The assessment and prediction of beer flavor stability. Proc Congr Eur Brew Conv 279–286
Schiestl RH, Gietz RD (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet 16(5–6):339–346
Shin KS, Kwon NJ, Kim YH, Park HS, Kwon GS, Yu JH (2009) Differential roles of the ChiB chitinase in autolysis and cell death of Aspergillus nidulans. Eukaryot Cell 8(5):738–746
Ueno K, Namiki Y, Mitani H, Yamaguchi M, Chibana H (2011) Differential cell wall remodeling of two chitin synthase deletants Deltachs3A and Deltachs3B in the pathogenic yeast Candida glabrata. FEMS Yeast Res 11(5):398–407. doi:10.1111/j.1567-1364.2011.00728.x
Wang J (2013) Autolysis evaluation of lager yeast using a complex parameter, 2013 ASBC annual meeting, http://www.asbcnet.org/meetings/proceedings/2013/oral.htm, No. 39, Accessed 14 June 2013
Wang JJ, Xiu PH (2010) Construction of amylolytic industrial brewing yeast strain with high glutathione content for manufacturing beer with improved anti-staling capability and flavor. J Microbiol Biotechn 20(11):1539–1545
Wang ZY, He XP, Liu N, Zhang BR (2008) Construction of self-cloning industrial brewing yeast with high-glutathione and low-diacetyl production. Int J Food Sci Tech 43(6):989–994
Wang ZY, Wang JJ, Liu XF, He XP, Zhang BR (2009) Recombinant industrial brewing yeast strains with ADH2 interruption using self-cloning GSH1+CUP1 cassette. FEMS Yeast Res 9(4):574–581
Wang JJ, Wang ZY, He XP, Zhang BR (2012) Integrated expression of the alpha-amylase, dextranase and glutathione gene in an industrial brewer’s yeast strain. World J Microb Biot 28(1):223–231. doi:10.1007/s11274-011-0811-6
Wang J, Shen N, Yin H, Liu C, Li Y, Li Q (2013) Development of industrial brewing yeast with low acetaldehyde production and improved flavor stability. Appl Biochem Biotechnol 169(3):1016–1025. doi:10.1007/s12010-012-0077-y
Xu YB, Li HP, Zhang JB, Song B, Chen FF, Duan XJ, Xu HQ, Liao YC (2010) Disruption of the chitin synthase gene CHS1 from Fusarium asiaticum results in an altered structure of cell walls and reduced virulence. Fungal Genet Biol 47(3):205–215
Yamaguchi M, Okada H, Namiki Y (2009) Smart specimen preparation for freeze substitution and serial ultrathin sectioning of yeast cells. J Electron Microsc 58(4):261–266
Zhang Y, Li Q, Shen W, Xie Y, Gu G (2008) Effects of Knockout ECM25/YJL201W gene in brewing yeast on beer flavor stability. Chin J Biotech 24(8):1420–1427 (In Chinese)
Acknowledgments
This study was financially supported by the program for New Century Excellent Talents in University of China (No. NCET-10-0453), the National High Technology Research and Development Program 863 (No. 2012AA021303), National Science Foundation (No. 31271919), National Science Foundation (No. 31301539), and Program of Introducing Talents of Discipline to Universities (No. 111-2-06).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Jj., Xu, Wn., Li, X. et al. Absence of fks1p in lager brewing yeast results in aberrant cell wall composition and improved beer flavor stability. World J Microbiol Biotechnol 30, 1901–1908 (2014). https://doi.org/10.1007/s11274-014-1617-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-014-1617-0