Abstract
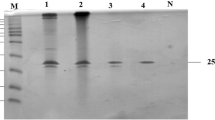
A xylanase gene, xyn-b39, coding for a multidomain glycoside hydrolase (GH) family 10 protein was cloned from the genomic DNA of the alkaline wastewater sludge of a paper mill. Its deduced amino acid sequence of 1,481 residues included two carbohydrate-binding modules (CBM) of family CBM_4_9, one catalytic domain of GH 10, one family 9 CBM and three S-layer homology (SLH) domains. xyn-b39 was expressed heterologously in Escherichia coli, and the recombinant enzyme was purified and characterized. Xyn-b39 exhibited maximum activity at pH 7.0 and 60 °C, and remained highly active under alkaline conditions (more than 80 % activity at pH 9.0 and 40 % activity at pH 10.0). The enzyme was thermostable at 55 °C, retaining more than 90 % of the initial activity after 2 h pre-incubation. Xyn-b39 had wide substrate specificity and hydrolyzed soluble substrates (birchwood xylan, beechwood xylan, oat spelt xylan, wheat arabinoxylan) and insoluble substrates (oat spelt xylan and wheat arabinoxylan). Hydrolysis product analysis indicated that Xyn-b39 was an endo-type xylanase. The K m and V max values of Xyn-b39 for birchwood xylan were 1.01 mg/mL and 73.53 U/min/mg, respectively. At the charge of 10 U/g reed pulp for 1 h, Xyn-b39 significantly reduced the Kappa number (P < 0.05) with low consumption of chlorine dioxide alone.



Similar content being viewed by others
References
Ali MK, Hayashi H, Karita S, Goto M, Kimura T, Sakka K, Ohmiya K (2001) Importance of the carbohydrate-binding module of Clostridium stercorarium Xyn10B to xylan hydrolysis. Biosci Biotechnol Biochem 65:41–47
Bai Y, Wang J, Zhang Z, Yang P, Shi P, Luo H, Meng K, Huang H, Yao B (2010) A new xylanase from thermoacidophilic Alicyclobacillus sp. A4 with broad-range pH activity and pH stability. J Ind Microbiol Biotechnol 37:187–194
Beg Q, Kapoor M, Mahajan L, Hoondal G (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56:326–338
Boraston A, McLean B, Kormos J, Alam M, Gilkes N, Haynes C, Tomme P, Kilburn D, Warren R (1999) Carbohydrate-binding modules: diversity of structure and function. Spec Publ R Soc Chem 246:202–211
Boraston A, Creagh A, Alam M, Kormos J, Tomme P, Haynes C, Warren RAJ, Kilburn DG (2001) Binding specificity and thermodynamics of a family 9 carbohydrate-binding module from Thermotoga maritima xylanase 10A. Biochemistry 40:6240–6247
Boraston A, Bolam D, Gilbert H, Davies G (2004) Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem J 382:769–781
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brady SF (2007) Construction of soil environmental DNA cosmid libraries and screening for clones that produce biologically active small molecules. Nat Protoc 2:1297–1305
Chanjuan L, Hong Y, Shao Z, Lin L, Huang X, Liu P, Wu G, Meng X, Liu Z (2009) Novel alkali-stable, cellulase-free xylanase from deep-sea Kocuria sp. Mn22. J Microbiol Biotechnol 19:873–880
Chen S, Qu Y, Liu X, Gao P (2000) Purification and properties of alkaline xylanase from Bacillus pumilus A-30. Chin J Biochem Mol Biol 16:698–701
Cheng YM, Hong TY, Liu C, Meng M (2009) Cloning and functional characterization of a complex endo-β-1,3-glucanase from Paenibacillus sp. Appl Microbiol Biotechnol 81:1051–1061
Christov L, Szakacs G, Balakrishnan H (1999) Production, partial characterization and use of fungal cellulase-free xylanases in pulp bleaching. Process Biochem 34:511–517
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23
Dhiman SS, Garg G, Mahajan R, Garg N, Sharma J (2009) Single lay out and mixed lay out enzymatic processes for biobleaching of kraft pulp. Bioresour Technol 100:4736–4741
Duarte MCT, Pellegrino ACA, Portugal EP, Ponezi AN, Franco TT (2000) Characterization of alkaline xylanases from Bacillus pumilus. Braz J Microbiol 31:90–94
Feng JX, Karita S, Fujino E, Fujno T, Kimura T, Sakka K, Ohmiya K (2000) Cloning, sequencing, and expression of the gene encoding a cell-bound multi-domain xylanase from Clostridium josui, and characterization of the translated product. Biosci Biotechnol Biochem 64:2614–2624
Gessesse A (1998) Purification and properties of two thermostable alkaline xylanases from an alkaliphilic Bacillus sp. Appl Environ Microbiol 64:3533–3535
Gessesse A, Gashe B (1997) Production of alkaline xylanase by an alkaliphilic Bacillus sp. isolated from an alkaline soda lake. J Appl Microbiol 83:402–406
Gunnarsson LC, Montanier C, Tunnicliffe RB, Williamson MP, Gilbert HJ, Karlsson EN, Ohlin M (2007) Novel xylan-binding properties of an engineered family 4 carbohydrate-binding module. Biochem J 406:209–214
Haarhoff J, Moes CJ, Cerff C, van Wyk WJ, Gerischer G, Janse BJH (1999) Characterization and biobleaching effect of hemicellulases produced by thermophilic fungi. Biotechnol Lett 21:415–420
Horikoshi K (1999) Alkaliphiles: some applications of their products for biotechnology. Microbiol Mol Biol Rev 63:735–750
Huang H, Wang G, Zhao Y, Shi P, Luo H, Yao B (2010) Direct and efficient cloning of full-length genes from environmental DNA by RT-qPCR and modified TAIL-PCR. Appl Microbiol Biotechnol 87:1141–1149
Hwang I, Lim H, Song H, Cho S, Chang J, Park N (2010) Cloning and characterization of a xylanase, KRICT PX1 from the strain Paenibacillus sp. HPL-001. Biotechnol Adv 28:594–601
Ito Y, Tomita T, Roy N, Nakano A, Sugawara-Tomita N, Watanabe S, Okai N, Abe N, Kamio Y (2003) Cloning, expression, and cell surface localization of Paenibacillus sp. strain W-61 xylanase 5, a multidomain xylanase. Appl Environ Microbiol 69:6969–6978
Kamal KB, Balakrishnan H, Rele M (2004) Compatibility of alkaline xylanases from an alkaliphilic Bacillus NCL (87-6-10) with commercial detergents and proteases. J Ind Microbiol Biotechnol 31:83–87
Kubata BB, Takamizawa K, Kawai K, Suzuki T, Horitsu H (1995) Xylanase IV, an exoxylanase of Aeromonas caviae ME-1 which produces xylotetraose as the only low-molecular-weight oligosaccharide from xylan. Appl Environ Microbiol 61:1666–1668
Kulkarni N, Shendye A, Rao M (1999) Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev 23:411–456
Li X, Jiang Z, Li L, Yang S, Feng W, Fan J, Kusakabe I (2005) Characterization of a cellulase-free, neutral xylanase from Thermomyces lanuginosus CBS 288.54 and its biobleaching effect on wheat straw pulp. Bioresour Technol 96:1370–1379
Li N, Meng K, Wang Y, Shi P, Luo H, Bai Y, Yang P, Yao B (2008) Cloning, expression, and characterization of a new xylanase with broad temperature adaptability from Streptomyces sp. S9. Appl Microbiol Biotechnol 80:231–240
Mangala S, Kittur F, Nishimoto M, Sakka K, Ohmiya K, Kitaoka M, Hayashi K (2003) Fusion of family VI cellulose binding domains to Bacillus halodurans xylanase increases its catalytic activity and substrate-binding capacity to insoluble xylan. J Mol Catal B Enzym 21:221–230
Mielenz JR (2001) Ethanol production from biomass: technology and commercialization status. Curr Opin Microbiol 4:324–329
Miller G (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Nakamura S, Wakabayashi K, Nakai R, Aono R, Horikoshi K (1993) Production of alkaline xylanase by a newly isolated alkaliphilic Bacillus sp. strain 41 M-1. World J Microbiol Biotechnol 9:221–224
Patel RN, Grabski AC, Jeffries TW (1993) Chromophore release from kraft pulp by purified Streptomyces roseiscleroticus xylanases. Appl Microbiol Biotechnol 39:405–412
Prade R (1996) Xylanases: from biology to biotechnology. Biotechnol Genet Eng Rev 13:101–131
Ratanakhanokchai K, Kyu KL, Tanticharoen M (1999) Purification and properties of a xylan-binding endoxylanase from alkaliphilic Bacillus sp. strain K-1. Appl Environ Microbiol 65:694–697
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291
Shirkolaee YZ, Talebizadeh A, Soltanali S (2008) Comparative study on application of T. lanuginosus SSBP xylanase and commercial xylanase on biobleaching of non wood pulps. Bioresour Technol 99:7433–7437
St. John FJ, Rice JD, Preston JF (2006) Paenibacillus sp. strain JDR-2 and XynA1: a novel system for methylglucuronoxylan utilization. Appl Environ Microbiol 72:1496–1506
Subramaniyan S, Prema P (2000) Cellulase-free xylanases from Bacillus and other microorganisms. FEMS Microbiol Lett 183:1–7
Takami H, Nakasone K, Takaki Y, Maeno G, Sasaki R, Masui N, Fuji F, Hirama C, Nakamura Y, Ogasawara N (2000) Complete genome sequence of the alkaliphilic bacterium Bacillus halodurans and genomic sequence comparison with Bacillus subtilis. Nucleic Acids Res 28:4317–4331
Torronen A, Rouvinen J (1997) Structural and functional properties of low molecular weight endo-1,4-β-xylanases. J Biotechnol 57:137–149
Usui K, Ibata K, Suzuki T, Kawai K (1999) A possible exo-xylanase of Aeromonas caviae ME-1 that produces exclusively xylobiose and xylotetraose from xylan. Biosci Biotechnol Biochem 63:1346–1352
Vicuña R, Escobar F, Osses M, Jara A (1997) Bleaching of eucalyptus kraft pulp with commercial xylanases. Biotechnol Lett 19:575–578
Viikari L, Kantelinen A, Sundquist J, Linko M (1994) Xylanases in bleaching: from an idea to the industry. FEMS Microbiol Rev 13:335–350
Waeonukul R, Pason P, Kyu KL, Sakka K, Kosugi A, Mori Y, Ratanakhanokchai K (2009) Cloning, sequencing, and expression of the gene encoding a multidomain endo-β-1, 4-xylanase from Paenibacillus curdlanolyticus B-6, and characterization of the recombinant enzyme. J Microbiol Biotechnol 19:277–285
Wong K, Tan L, Saddler JN (1988) Multiplicity of β-1,4-xylanase in microorganisms: functions and applications. Microbiol Mol Biol Rev 52:305–317
Yang V, Zhuang Z, Elegir G, Jeffries T (1995) Alkaline-active xylanase produced by an alkaliphilic Bacillus sp. isolated from kraft pulp. J Ind Microbiol Biotechnol 15:434–441
Zhao G, Ali E, Sakka M, Kimura T, Sakka K (2006) Binding of S-layer homology modules from Clostridium thermocellum SdbA to peptidoglycans. Appl Microbiol Biotechnol 70:464–469
Zhao Y, Luo H, Meng K, Shi P, Wang G, Yang P, Yuan T, Yao B (2011) A xylanase gene directly cloned from the genomic DNA of alkaline wastewater sludge showing application potential in the paper industry. Appl Biochem Biotechnol 165:35–46
Acknowledgments
This research was supported by the National Science and Technology Support Program (2011BADB02) and the China Modern Agriculture Research System (CARS-42) and the National “948” project (2011-G7-4).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yanyu Zhao and Kun Meng contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.

11274_2012_1186_MOESM1_ESM.jpg
Fig. S1 Amino acid sequence alignment of Xyn-b39 (JN020645) with two GH 10 xylanases from Paenibacillus sp. W-61 (BAC45001.1) and Thermobacillus composti KWC4 (EGZ39961). (JPEG 182 kb)
Rights and permissions
About this article
Cite this article
Zhao, Y., Meng, K., Luo, H. et al. Molecular and biochemical characterization of a new alkaline active multidomain xylanase from alkaline wastewater sludge. World J Microbiol Biotechnol 29, 327–334 (2013). https://doi.org/10.1007/s11274-012-1186-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-012-1186-z