Abstract
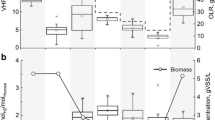
The ability of Acidithiobacillus caldus to grow aerobically using pyruvate, acetate, citrate, 2-ketoglutarate, succinate, and malate as either an electron donor and carbon source (heterotrophic growth), or as a carbon source when potassium tetrathionate was added as an electron donor (mixotrophic growth), was tested in chemostat cultures. Under both heterotrophic and mixotrophic conditions, organic acids were added to a sub-lethal concentration (50 μM). Under mixotrophic conditions, potassium tetrathionate was added to an excess concentration (10 mM). No cell growth was observed under heterotrophic conditions; however, effluent cell concentrations increased over threefold when pyruvate was coupled with potassium tetrathionate. Under these conditions, the effluent pyruvate concentration was reduced to below the detection limit (2 μM), and oxygen consumption increased by approximately 100%. Although pyruvate provided a carbon source in these experiments, ambient carbon dioxide was also available to the cells. To test whether At. caldus could grow mixotrophically using pyruvate as a sole carbon source and potassium tetrathionate as an electron donor, cells were batch cultured in a medium free of dissolved inorganic carbon, and with no carbon dioxide in the headspace. These experiments showed that At. caldus was able to convert between 65 ± 8 and 82 ± 15% of the pyruvate carbon to cellular biomass, depending on the initial pyruvate concentrations. This work is the first to identify a defined organic-carbon source, other than glucose, that At. caldus can assimilate. This has important implications, as mixotrophic and heterotrophic activity has been shown to increase mineral leaching in acidic systems.




Similar content being viewed by others
References
Aston JA, Apel WA, Lee BD, Peyton BM (2009) Toxicity of select organic acids to the slightly thermophilic acidophile Acidithiobacillus caldus. Environ Toxicol Chem 28:279–286
Borischewski RM (1967) Keto acids as growth-limiting factors in autotrophic growth of Thiobacillus thiooxidans. J Bacteriol 93:597–599
Burckhard SR, Schwab AP, Banks MK (1995) The effects of organic acids on the leaching of heavy metals from mine tailings. J Hazard Mat 41:135–145
Dopson M, Lindstrom EB (1999) Potential role of Thiobacillus caldus in arsenopyrite bioleaching. Appl Environ Microbiol 65:36–40
Dopson M, Lindstrom EB, Hallberg KB (2002) ATP generation during reduced inorganic sulfur compound oxidation by Acidithiobacillus caldus is exclusively due to electron transport phosphorylation. Extremophiles 6:123–129
Edwards KJ, Bond PL, Banfield JF (2000) Characteristics of attachment and growth of Thiobacillus caldus on sulphide minerals: a chemotactic response to sulphur minerals? Environ Microbiol 2:324–332
Fu B, Zhou H, Zhang R, Qiu G (2008) Bioleaching of chalcopyrite by pure and mixed cultures of Acidithiobacillus spp. and Leptospirillum ferriphilum. Int J Biodeterior Biodegradation 62:109–115
Gu XY, Wong JWC (2004) Identification of inhibitory substances affecting bioleaching of heavy metals from anaerobically digested sewage sludge. Eniviron Sci Technol 38:2934–2939
Gu XY, Wong JWC (2007) Degradation of inhibitory substances by heterotrophic microorganisms during bioleaching of heavy metals from anaerobically digested sewage sludge. Chemosphere 69:311–318
Hallberg KB, Lindstrom EB (1994) Characterization of Thiobacillus caldus sp. Nov., a moderately thermophilic acidophile. Microbiology 140:3451–3456
Hallberg KB, Lindstrom EB (1996) Multiple serotypes of the moderate thermophile Thiobacillus caldus, a limitation of immunological assays for biomining microorganisms. Appl Environ Microbiol 62:4243–4246
Ingledew WJ, Poole RK (1982) Thiobacillus ferrooxidans: the bioenergetics of an acidophilic chemolithotroph. Biochem Biophys Acta 683:89–117
Marchland EA, Silverstein J (2003) The role of enhanced heterotrophic bacterial growth on iron oxidation by Acidithiobacillus ferrooxidans. Geomicrobiol J 20:231–244
Matin A (1978) Organic nutrition of chemolithotrophic bacteria. Annu Rev Microbiol 32:433–468
McGuire MM, Edwards KJ, Banfield JF, Hamers RJ (2001) Kinetics, surface chemistry, and structural evolution of microbially mediated sulfide mineral dissolution. Geochem Cosmochim Acta 65:1243–1258
Okibe N, Gericke M, Hallberg KB, Johnson DB (2003) Enumeration and characterization of acidophilic microorganisms isolated for a pilot plant stirred-tank bioleaching operation. Appl Environ Microbiol 69:1936–1943
Olson GJ, Brierley JA, Brierley CL (2003) Bioleaching review. Part B: progress in bioleaching: applications of microbial processes by the mineral industries. Appl Microbiol Biotechnol 63:249–257
Pronk JT, Meesters PJW, van Dijken JP, Bos P, Kuenen JG (1989) Heterotrophic growth of Thiobacillus acidophilus in batch and chemostat cultures. Microbiology 153:392–398
Pronk JT, Mejer WM, Hazeu W, van Dijken JP, Bos P, Kuenen JG (1991) Growth of Thiobacillus thiooxidans on formic acid. Appl Environ Microbiol 57:2057–2062
Rawlings DE (2002) Heavy metal mining using microbes. Annu Rev Microbiol 56:65–91
Richard HT, Foster JW (2003) Acid resistance in Escherichia coli. Adv Appl Microbiol 52:167–184
Schnaitman C, Lundgren D (1965) Organic compounds in the spent medium of Ferrobacillus ferrooxidans. Can J Microbiol 1:23–27
Temple KL, Colmer AR (1951) The autotrophic oxidation of iron by an new bacterium, Thiobacillus ferrooxidans. J Bacteriol 62:605–611
Tuttle JH, Dugan PR, Apel WA (1977) Leakage of cellular material from Thiobacillus ferrooxidans in the presence of organic acids. Appl Environ Microbiol 33:459–469
Valdes J, Pedroso I, Quatrini R, Holmes DS (2008) Comparative genome analysis of Acidithiobacillus ferrooxidans, A. thiooxidans and A. caldus: insights into their metabolism and ecophysiology. Hydrometallurgy 94:180–184
Valdes J, Quatrini R, Hallberg K, Dopson M, Valenzuela PDT, Holmes DS (2009) Draft genome sequence of the extremely acidophilic bacterium Acidithiobacillus caldus ATCC 51756 reveals metabolic versatility in the genus Acidithiobacillus. J Bact 191:5877–5878
Waksman SA, Joffe JS (1922) Microorganisms concerned in the oxidation of sulfur in the soil II. Thiobacillus thiooxidans, a new sulfuroxidizing organism isolated from the soil. J Bacteriol 7:239–256
Yilmaz EI (2003) Metal tolerance and biosorption capacity of Bacillus circulans strain EB1. Res Microbiol 154:409–415
Zhou QG, Bo F, Bo ZH, Xi L, Jian G, Fei LF, Hau CH (2007) Isolation of a strain of Acidithiobacillus caldus and its role in bioleaching of chalcopyrite. World J Microbiol Biotechnol 23:1217–1225
Acknowledgments
This work was supported in part through the Idaho National Laboratory Directed Research and Development program under Department of Energy Idaho Operations Office Contract DE-AC07-05ID14517. The authors also thank the Montana Experimental Program to Stimulate Competitive Research and the National Science Foundation Integrated Graduate Education Research Training program for financial support (grant # DGE-0654336), as well as the Department of Chemical and Biological Engineering and Center for Biofilm Engineering at Montana State University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aston, J.E., Apel, W.A., Lee, B.D. et al. Growth effects and assimilation of organic acids in chemostat and batch cultures of Acidithiobacillus caldus . World J Microbiol Biotechnol 27, 153–161 (2011). https://doi.org/10.1007/s11274-010-0441-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0441-4