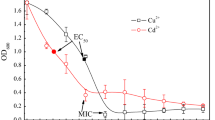
Abstract
Biosorptive capacity of Pb(II), Cd(II) and Cu(II) by lyophilized cells of Pseudomonas stutzeri was investigated based on Langmuir and Freundlich isotherms. Biosorptive capacity for Pb(II), Cd(II) and Cu(II) decreased with an increase of metal concentration, reaching 142, 43.5 and 36.2 mg/g at initial concentration of 300 mg/l, respectively. Biosorption capacity for metal ions increased with increasing pH. The optimum pH for biosorption rate of Cd(II) and Cu(II) were 5.0, and 6.0 for Pb(II) biosorption. The experimental data showed a better fit with the Langmuir model over the Freundlich model for metal ions throughout the range of initial concentrations. The maximum sorptive capacity (q max) obtained from the Langmuir equation for Pb(II), Cd(II) and Cu(II) were 153.3 (r 2 = 0.998), 43.86 (r 2 = 0.995), and 33.16 (r 2 = 0.997) for metal ions, respectively. The selectivity order for metal ions towards the biomass of P. stutzeri was Pb(II) > Cd(II) > Cu(II) for a given initial metal ions concentration. The interactions between heavy metals and functional groups on the cell wall surface of bacterial biomass were confirmed by FTIR analysis. The results of this study indicate the possible removal of heavy metals from the environment by using lyophilized cells of P. stutzeri.







Similar content being viewed by others
Abbreviations
- C i :
-
Initial metal concentration (mg/l)
- C e :
-
Equilibrium concentration (mg/l)
- q e :
-
Specific metal biosorption
- q max :
-
Maximum adsorption capacity of the metal ion (mg/g)
- b :
-
Ratio of adsorption rate constant to desorption rate constant (l/mg)
- V :
-
Volume of metal solution (l)
- M :
-
Mass of sorbent (g)
- K f :
-
Sorptive capacity
- n :
-
Sorptive intensity
- FTIR:
-
Fourier Transform Infrared Spectroscopy
- R 2 :
-
Regression coefficient
- SE:
-
Standard error
References
Acosta MP, Valdman E, Leite SGF, Battaglini F, Ruzal SM (2005) Biosorption of copper by Paenibacillus polymyxa cells and their exoploysaccharide. World J Microbiol Biotechnol 21:1157–1163. doi:10.1007/s11274-005-0381-6
Chen C, Wang J (2007) Influence of metal ionic characteristics on their biosorption capacity by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 74:911–917. doi:10.1007/s00253-006-0739-1
Cho JS, Hur JS, Kang BH, Kim PJ, Sohn BK, Lee HJ, Jung YK, Heo JS (2001) Biosorption of copper by immobilized biomass of Pseudomonas stutzer. J Microbiol Biotechnol 11:964–972
Eccles H (1999) Treatment of metal-contaminated wastes: why select a biological process? Trends Biotechnol 17:462–465. doi:10.1016/S0167-7799(99)01381-5
Fowle DA, Fein JB (1999) Competitive adsorption of metal cations onto two gram positive bacteria: testing the chemical equilibrium model. Geochim Cosmochim Acta 63:3059–3067. doi:10.1016/S0016-7037(99)00233-1
Gabr RM, Hassan SHA, Shoreit AAM (2008) Biosorption of lead and nickel by living and non-living cells of Pseudomonas aeruginosa ASU 6a. Int Biodeterior Biodegradation 62:195–203. doi:10.1016/j.ibiod.2008.01.008
Horsfall JM, Spiff AI (2005) Effect of metal ion concentration on the biosorption of Pb2+ and Cd2+ by Caladium bicolor (wild cocoyam). Afr J Biotechnol 4:191–196
Kadukova J, Vircikova E (2005) Comparison of differences between copper bioaccumulation and biosorption. Environ Int 31:227–232. doi:10.1016/j.envint.2004.09.020
Komy ZR, Gabar RM, Shoriet AAM, Mohammed RM (2006) Characterization of acidic sites of Pseudomonas biomass capable of binding protons and cadmium and removal of cadmium via biosorption. World J Microbiol Biotechnol 22:975–982. doi:10.1007/s11274-006-9143-3
Lodeiro P, Barriada JL, Herrero R, Sastre de Vicente ME (2006) The marine macroalga Cystoseira baccata as biosorbent for cadmium(II) and lead(II) removal: kinetic and equilibrium studies. Environ Pollut 142:264–273. doi:10.1016/j.envpol.2005.10.001
Lu WB, Shi JJ, Wang CH, Chang JS (2006) Biosorption of lead, copper and cadmium by an indigenous isolate Enterobacter sp. J1 possessing high heavy-metal resistance. J Hazard Mater 134:80–86. doi:10.1016/j.jhazmat.2005.10.036
Norton L, Baskaran K, McKenzie T (2004) Biosorption of zinc from aqueous solutions using biosolids. Adv Environ Res 8:629–635. doi:10.1016/S1093-0191(03)00035-2
Ok YS, Yang JE, Zhang YS, Kim SJ, Chung DY (2007) Heavy metal adsorption by a formulated zeolite-Portland cement mixture. J Hazard Mater 147:91–96. doi:10.1016/j.jhazmat.2006.12.046
Pardo R, Herguedas M, Barrado E, Vega M (2003) Biosorption of cadmium, copper, lead and zinc by inactive biomass of Pseudomonas putida. Anal Bioanal Chem 376:26–32
Sar P, Kazy SK, Asthana RK, Singh SP (1999) Metal adsorption and desorption by lyophilized Pseudomonas aeruginosa. Int Biodeterior Biodegradation 44:101–110. doi:10.1016/S0964-8305(99)00064-5
Singleton P, Sainsbury D (1987) Dictionary of microbiology and molecular biology. Wiley, New York, p 721
Smith JL, Collins HP (2007) Management of organisms and their processes in soils. In: Eldor AP (ed) Soil microbiology, ecology, and biochemistry. Elsevier, Burlington, pp 389–430
Tunali S, Çabuk A, Akar T (2006) Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem Eng J 115:203–211. doi:10.1016/j.cej.2005.09.023
Volesky B (1990) Biosorption of heavy metals. CRC Press, Boca Raton, pp 7–44
Zouboulis AI, Loukidou MX, Matis KA (2004) Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process Biochem 39:909–919. doi:10.1016/S0032-9592(03)00200-0
Acknowledgments
This study was partially supported by a grant from the Research Institute of Agricultural Science, Kangwon National University, and 2006 grant from Kangwon National University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, S.E., Hassan, S.H.A. & Joo, J.H. Biosorption of heavy metals by lyophilized cells of Pseudomonas stutzeri . World J Microbiol Biotechnol 25, 1771–1778 (2009). https://doi.org/10.1007/s11274-009-0075-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-0075-6