Abstract
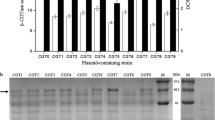
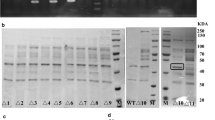
The polyhydroxyalkanoate biosynthesis gene locus from Bacillus thuringiensis R1 was isolated, cloned and analyzed at the molecular level. We found that a ∼5 kb SacI–ClaI digested fragment of genomic DNA from B. thuringiensis R1 encoding the PHA synthesising genes, conferred PHA producing ability to E. coli. The fragment was sequenced and found to be of 4787 bp with five open reading frames. Sequence alignment with closely related species of Bacillus in the existing database revealed that the ORFs correspond to phaP, phaQ, phaR, phaB and phaC genes. However, E. coli harboring phaP, phaQ, phaR, phaB and phaC locus produced very low PHA. Furthermore, complementation of the locus with phaA from Ralstonia eutropha increased the PHA production in the recombinant E. coli from 3.0% to 24% of cell dry mass. The putative promoter regions and ribosome binding sites were identified for each of the gene. Conserved domains for PHA synthase and aceto-acetyl-coA reductase were also identified. We hence conclude that the PHA operon of Bacillus thuringiensis R1 consists of phaP, phaQ, phaR, phaB, phaC and complementation of the same with phaA is accountable for its high PHA production.


Similar content being viewed by others
References
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role and industrial uses of Bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Borodovsky M, McIninch J (1993) GeneMark: parallel gene recognition for both DNA strands. Computers & Chemistry 17:123–133
Braunegg G, Lefebvre G, Genser KF (1998) Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J Biotechnol 65:127–161
Heikinheimo P, Goldman A, Jeffries C, Ollis DL (1999) Of barn owls and bankers: a lush variety of α/β hydrolases, Structure with Folding & Design. Structure 7:141–146
Jia Y, Kappock TJ, Frick T, Sinskey AJ, Stubbe J (2000) Lipases provide a new mechanistic model for polyhydroxybutyrate (PHB) synthases: characterization of the functional residues in Chromatium vinosum PHB synthase. Biochemistry 39:3927–3936
Lee SY (1996) Bacterial Polyhydroxyalkanoates. Biotechnol Bioeng 49:1–14
Lee TR, Lin JS, Wang SS, Shaw GC (2004) PhaQ, a new class of poly-beta-hydroxybutyrate (phb)-responsive repressor, regulates phaQ and phaP (phasin) expression in Bacillus megaterium through interaction with PHB. J Bacteriol 186:3015–3021
McCool GJ, Cannon MC (1999) Polyhydroxyalkanoate inclusion body-associated proteins and coding region in Bacillus megaterium. J Bacteriol 181:585–592
McCool GJ, Cannon MC (2001) PhaC and PhaR are required for polyhydroxyalkanoic acid synthase activity in Bacillus megaterium. J Bacteriol 183:4235–4243
McCool GJ, Fernandez T, Cannon MC (1996) Polyhydroxyalkanoate inclusion body-growth and proliferation in Bacillus megaterium. FEMS Microbiol Lett 138:41–48
Müh U, Sinskey AJ, Kirby DP, Lane WS, Stubbe J (1999) PHA synthase from Chromatium vinosum: Cys 149 is involved in covalent catalysis. Biochemistry 38:826–837
Oldenburg GT, Nishina K, Stephanopoulos G (2000) Identification and analysis of the polyhydroxyalkanoate-Specific β-ketothiolase and acetoacetyl-CoA reductase genes in Cyanobacterium Synechocystis sp. strain PCC6803. Appl Environ Microbiol 66:4440–4448
Ploux O, Masamune S, Walsh CT (1988) The NADPH-linked acetoacetyl-CoA reductase from Zoogloea ramigera. Characterization and mechanistic studies of the cloned enzyme over-produced in Escherichia coli. Eur J Biochem 174:177–182
Pospiech A, Neumann B (1995) A versatile quick-prep of Genomic DNA from gram positive bacteria. Trends Genet 11:217–218
Pötter M, Steinbüchel A (2005) Poly(3-hydroxybutyrate) Granule-Associated Proteins: Impacts on Poly(3-hydroxybutyrate) Synthesis and Degradation. Biomacromolecules 6:552–560
Pötter M. Madkour MH, Mayer F, Steinbüchel A (2002) Regulation of phasin expression and polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha H16. Microbiology 148:2413–2426
Qi Q, Rehm BHA (2001) Polyhydroxybutyrate biosynthesis in Caulobacter crescentus: molecular characterization of the polyhydroxybutyrate synthase. Microbiology 147:3353–3358
Rehm BHA (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33
Rehm BHA, Steinbüchel A (2001) Bioploymers. In: Steinbüchel A, Doi Y (eds) PHA synthases—the key enzyme of PHA synthesis. Wiley-VCH, Heidelberg, pp 173–180
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning; A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Satoh Y, Minamoto N, Tajima K. Munekata M (2002) Polyhydroxyalkanoate synthase from Bacillus sp INT005 is composed of PhaC and PhaR. J Biosci Bioeng 94:343–350
Solaiman DK, Ashby RD (2005) Rapid genetic characterization of Poly(hydroxyalkanoate) synthase and its applications. Biomacromolecules 6:532–537
Steinbüchel A (1991) Biomaterials. In: Byrom D (ed) Novel materials from biological sources. MacMillan Publishers, Basingstoke, pp. 123–213
Steinbüchel A, Hein S (2001) Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganism. Adv Biochem Eng Biotechnol 71:82–119
York GM, Stubbe J, Sinskey AJ (2002) The Ralstonia eutropha PhaR protein couples synthesis of the PhaP phasin to the presence of polyhydroxybutyrate in cells and promotes polyhydroxybutyrate production. J Bacteriol 184:59–66
Acknowledgements
Rohini D. Desetty thanks University Grants Commission, INDIA for the financial support during the work period. We are grateful to Dr. Satoh (Hokkaido University, Japan) for providing us with the primers (P5 and P6) and the plasmid pSRAB. We thank the reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Desetty, R.D., Mahajan, V.S., Khan, B.M. et al. Isolation and heterologous expression of PHA synthesising genes from Bacillus thuringiensis R1. World J Microbiol Biotechnol 24, 1769–1774 (2008). https://doi.org/10.1007/s11274-008-9669-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9669-7