Summary
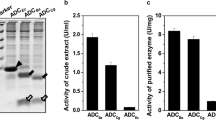
To understand the structure–function relationships of Bacillus stearothermophilus leucine aminopeptidase II (LAPII), each of the four conserved asparagine residues was replaced with leucine, aspartate, and lysine respectively by site-directed mutagenesis. The over-expressed wild-type and mutant enzymes with an apparent molecular mass of approximately 44.5 kDa were purified to homogeneity by nickel-chelate chromatography. Substitution of Asn-245, Asn-335, and Asn-341 with Lys generated variants with a dramatic loss of LAP activity. Kinetic analysis of Asn-373 variants with p-leucine-nitroanilide as the substrate revealed an increase in k cat with no significant change in K m, leading to a more than 2-fold increase in the catalytic efficiency. Thermostability assays showed that replacement of Asn-335, Asn-341, and Asn-373 by aspartic acid markedly increased the half-life of the enzyme at 70 °C, indicating that the deamination of these residues may have a deleterious effect on LAPII.
Similar content being viewed by others
References
T.J. Ahern J.I. Casal G.A. Petsko A.M. Klibanov (1987) ArticleTitleControl of oligomeric enzyme thermostability by protein engineering Proceedings of the National Academy of Sciences of the United States of America 84 675–679 Occurrence Handle1:CAS:528:DyaL2sXktVehs7k%3D
B. Chevrier C. Schalk H. D’Orchymont J.M. Rondeau D. Moras C. Tarnus (1994) ArticleTitleCrystal structure of Aeromonas proteolytica aminopeptidase: a prototypical member of the co-catalytic zinc enzyme family Structure 2 283–291 Occurrence Handle10.1016/S0969-2126(00)00030-7 Occurrence Handle1:CAS:528:DyaK2MXhslyksQ%3D%3D
M.C. Chi W.M. Chou W.H. Hsu L.L. Lin (2004) ArticleTitleIdentification of amino acid residues essential for the catalytic reaction of Bacillus kaustophilus leucine aminopeptidase Bioscience, Biotechnology, and Biochemistry 68 1794–1797 Occurrence Handle1:CAS:528:DC%2BD2cXnsVKntbg%3D
T.E. Creighton (1993) Proteins: Structure and Molecular Properties WH Freeman and Co. New York 9–10
G.J. Davies S.J. Gamblin J.A. Littlechild H.C. Watson (1993) ArticleTitleThe structure of a thermally stable 3-phosphoglycerate kinase and a comparison with its mesophilic equivalent Proteins 15 283–289 Occurrence Handle10.1002/prot.340150306 Occurrence Handle1:CAS:528:DyaK3sXhvVKru7k%3D
L.F. Delboni S.C. Mande F. Rentier-Delrue V. Mainfroid S. Turley F.M. Vellieux J.A. Martial W.G. Hol (1995) ArticleTitleCrystal structure of recombinant triosephosphate isomerase from Bacillus stearothermophilus: an analysis of potential thermostability factors in six isomerases with known 3-dimensional structures points to the importance of hydrophobic interactions Protein Sciences 4 2594–2604 Occurrence Handle1:CAS:528:DyaK2MXpslOnt7w%3D
N. Declerck M. Machius G. Wiegand R. Huber C. Gaillardin (2000) ArticleTitleProbing structural determinants specifying high thermostability in Bacillus licheniformis α-amylase Journal of Molecular Biology 301 1041–1057 Occurrence Handle10.1006/jmbi.2000.4025 Occurrence Handle1:CAS:528:DC%2BD3cXmtVKlsrg%3D
V.G. Eijsink J.R. Zee Particlevan der B. Burg Particlevan den G. Vriend G. Venema (1991) ArticleTitleImproving the thermostability of the neutral protease of Bacillus stearothermophilus by replacing a buried asparagine by leucine FEBS Letters 282 13–16 Occurrence Handle10.1016/0014-5793(91)80434-5 Occurrence Handle1:CAS:528:DyaK3MXksVGjtLk%3D
M. Grdisa L. Vitale (1991) ArticleTitleTypes and localization of aminopeptidases in different human blood cells International Journal of Biochemistry 23 339–345 Occurrence Handle10.1016/0020-711X(91)90072-U Occurrence Handle1:CAS:528:DyaK3MXnvFantw%3D%3D
R. Gilboa A. Spungin-Bialik G. Wohlfahrt D. Schomburg S. Blumberg G. Shoham (2001) ArticleTitleInteractions of Streptomyces griseus aminopeptidase with amino acid reaction products and their implications toward a catalytic mechanism Proteins 44 490–504 Occurrence Handle10.1002/prot.1115 Occurrence Handle1:CAS:528:DC%2BD3MXmt1Sjtb8%3D
A.L. Goldberg P. Cascio T. Saric K.L. Rock (2002) ArticleTitleThe importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides Molecular Immunology 39 147–164 Occurrence Handle10.1016/S0161-5890(02)00098-6 Occurrence Handle1:CAS:528:DC%2BD38XmsVWqtLc%3D
Y.Q. Gu V.P. Pautot F.M. Holzer L.L. Walling (1996) ArticleTitleA complex array of proteins related to the multimeric leucine aminopeptidase of tomato Plant Physiology 110 1257–1266 Occurrence Handle1:CAS:528:DyaK28XitlWjt7k%3D
Y.Q. Gu L.L. Walling (2002) ArticleTitleIdentification of residues critical for activity of the wound-induced leucine aminopeptidase (LAP-A) of tomato European Journal of Biochemistry 269 1630–1640 Occurrence Handle1:CAS:528:DC%2BD38XisVKisbk%3D
K.S. Hui M. Hui A. Lajtha M. Saito (1990) ArticleTitleCellular localization of puromycin-sensitive aminopeptidase isozymes Neurochemical Research 15 1147–1151 Occurrence Handle10.1007/BF01208573 Occurrence Handle1:CAS:528:DyaK3MXisVCmtLg%3D
U. Jankiewicz W. Bielawski (2003) ArticleTitleThe properties and functions of bacterial aminopeptidases Acta Microbiologica Polonica 52 217–231 Occurrence Handle1:CAS:528:DC%2BD2cXltVCn
Kamphius, J., Meijer, E.M., Boesten, W.H.J., Broxterman, Q.B., Kaptein, B., Hermes, H.F.M. & Schoemaker, H.E. (1992). Production of natural and synthetic L- and D-amino acids by aminopeptidases and amino amidases, . In J.D. Rozzell, & F. Wagner eds. (pp. 178–206). John Wiley & Sons, New York ISBN 3-44-6156972.
C.A. Kelly M. Nishiyama Y. Ohnishi T. Beppu J.J. Birktoft (1993) ArticleTitleDeterminants of protein thermostability observed in the 1.9-Å crystal structure of malate dehydrogenase from the thermophilic bacterium Thermus flavus Biochemistry 32 3913–3922 Occurrence Handle10.1021/bi00066a010 Occurrence Handle1:CAS:528:DyaK3sXhvVKrtLs%3D
H. Kim S.K. Burley W.N. Lipscomb (1993) ArticleTitleRe-refinement of the X-ray crystal structure of bovine lens leucine aminopeptidase complexed with bestatin Journal of Molecular Biology 230 722–734 Occurrence Handle1:CAS:528:DyaK3sXisFSltrY%3D
H. Kim W.N. Lipscomb (1993) ArticleTitleDifferentiation and identification of the two catalytic metal binding sites in bovine lens leucine aminopeptidase by X-ray crystallography Proceedings of the National Academy of Sciences of the United States of America 90 5006–5010 Occurrence Handle1:CAS:528:DyaK3sXksVChs78%3D
I. Korndörfer B. Steipe R. Huber A. Tomschy R. Jaenicke (1995) ArticleTitleThe crystal structure of holo-glyceraldehyde-3-phosphate dehydrogenase from the hyperthermophilic bacterium Thermotoga maritima at 2.5 Å resolution Journal of Molecular Biology 246 511–521
L.Y. Kuo G.Y. Hwang Y.J. Lai S.L. Yang L.L. Lin (2003) ArticleTitleOverexpression, purification, and characterization of the recombinant leucine aminopeptidase II of Bacillus stearothermophilus Current Microbiology 47 40–45 Occurrence Handle1:CAS:528:DC%2BD3sXks1OrtrY%3D
U.K. Laemmli (1970) ArticleTitleCleavage of structural proteins during the assembly of the head of bacteriophage T4 Nature (Lodon) 227 680–685 Occurrence Handle1:CAS:528:DC%2BD3MXlsFags7s%3D
W.T. Lowther B.W. Matthews (2002) ArticleTitleMetalloaminopeptidases: common functional themes in disparate structural surroundings Chemical Reviews 102 4581–4607 Occurrence Handle10.1021/cr0101757 Occurrence Handle1:CAS:528:DC%2BD38XosFSgu7c%3D
Manchenko, G.P. (1994) Handbook of Detection of Enzymes on Electrophoresis Gels. London: CRC Press. ISBN 0-84931-257-4, pp. 3.4.11.1
B.W. Matthews (1993) ArticleTitleStructural and genetic analysis of protein stability Annual Review of Biochemistry. 62 249–278
M. Nelson M. McClelland (1992) ArticleTitleUse of DNA methyltransferase/endonuclease enzyme combinations for megabase mapping of chromosomes Methods in Enzymology 216 279–303 Occurrence Handle1:CAS:528:DyaK3sXkt1Wnt7o%3D Occurrence Handle10.1016/0076-6879(92)16027-H
V. Pautot F.M. Holzer B. Reisch L.L. Walling (1993) ArticleTitleLeucine aminopeptidase: an inducible component of the defense response in Lycopersicon esculentum (tomato) Proceedings of the National Academy of Sciences of the United States of America 90 9906–9910 Occurrence Handle1:CAS:528:DyaK2cXhtVahur4%3D
M. Ramirez G. Arechaga S. Garcia B. Sanchez P. Lardeli J.M. Gandarias (1990) ArticleTitleSoluble and membrane-bound leucyl- and arginyl-aminopeptidase activities in subcellular fractions of young and adult rat brains Revista Espanola de Fisiologia 46 393–397 Occurrence Handle1:CAS:528:DyaK3MXmsFaksL0%3D
M.B. Rao A.M. Tanksale M.S. Ghatge V.V. Desphade (1998) ArticleTitleMolecular and biotechnological aspects of microbial proteases Microbiology and Molecular Biology Reviews 62 597–635 Occurrence Handle1:CAS:528:DyaK1cXmtFOjurs%3D
S.L. Roderick B.W. Matthews (1993) ArticleTitleStructure of the cobalt-dependent methionine aminopeptidase from Escherichia coli: a new type of proteolytic enzyme Biochemistry 32 3907–3912 Occurrence Handle10.1021/bi00066a009 Occurrence Handle1:CAS:528:DyaK3sXitVynsL8%3D
S. Rowsell R.A. Pauptit A.D. Tucker R.G. Melton D.M. Blow P. Brick (1997) ArticleTitleCrystal structure of carboxypeptidase G2, a bacterial enzyme with applications in cancer therapy Structure 5 337–347 Occurrence Handle10.1016/S0969-2126(97)00191-3 Occurrence Handle1:CAS:528:DyaK2sXisVyqu7s%3D
J. Sambrook D.W. Russell (2001) Molecular Cloning: A Laboratory Manual Cold Spring Harbor Laboratory Press, Cold-Spring Harbor New York 1.31–1.125
N. Sträter W.N. Lipscomb (1995) ArticleTitleTransition state analogue L-leucinephosphonic acid bound to bovine leucine aminopeptidase: X-ray structure at 1.65 Å resolution in a new crystal form Biochemistry 34 9200–9210
N. Sträter D.J. Sherratt S.D. Colloms (1999) ArticleTitleX-ray structure of aminopeptidase A from Escherichia coli and a model for the nucleoprotein complex in Xer site-specific recombination EMBO Journal 18 4513–4522
L. Terenius J. Sandin T. Sakurada (2000) ArticleTitleNociceptin/orphanin FQ metabolism and bioactive metabolites Peptides 21 919–922 Occurrence Handle10.1016/S0196-9781(00)00228-X Occurrence Handle1:CAS:528:DC%2BD3cXms1WrsLk%3D
C. Vieille J.M. Hess R.M. Kelly J.G. Zeikus (1995) ArticleTitlexylA cloning and sequencing and biochemical characterization of xylose isomerase from Thermotoga neapolitana Applied and Environmental Microbiology 61 1867–1875 Occurrence Handle1:CAS:528:DyaK2MXlsFyktL8%3D
C. Vieille G.J. Zeikus (2001) ArticleTitleHyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability Microbiology and Molecular Biology Reviews 65 1–43 Occurrence Handle10.1128/MMBR.65.1.1-43.2001 Occurrence Handle1:CAS:528:DC%2BD3MXisFyms74%3D
M.C.J. Wilce C.S. Bond N.E. Dixon H.C. Freeman J.M. Guss P.E. Lilly J.A. Wilce (1998) ArticleTitleStructure and mechanism of a proline-specific aminopeptidase from Escherichia coli Proceedings of the National Academy of Sciences of the United States of America 95 3472–3477 Occurrence Handle10.1073/pnas.95.7.3472 Occurrence Handle1:CAS:528:DyaK1cXitlKjsrk%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, RS., Yang, SL., Hua, YW. et al. Directed Mutagenesis of the Conserved Asparagine Residues of Bacillus Stearothermophilus Leucine Aminopeptidase II. World J Microbiol Biotechnol 21, 1477–1482 (2005). https://doi.org/10.1007/s11274-005-7023-x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11274-005-7023-x