Abstract
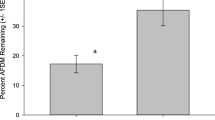
Restoration of mined Restionaceae-dominated peat bogs in northern New Zealand is currently initiated by establishing native vegetation cover to minimise erosion of the remaining peat. The relative effects of various restoration techniques on litter decomposition and microbial activity within experimental litter bags were investigated in a restoration trial established on a mined peat surface. Decomposition and microbial activity of litter were compared between four different restoration treatments: direct transfer of intact habitat ‘islands’; the addition of processed peat with seed; the addition of processed peat with no seed; and recently mined peat surface (a ‘do nothing’ restoration option), with the four treatments replicated at each of five distances from an undisturbed peat bog. Treatments were compared with an undisturbed peat bog (control). Litter decomposition and associated microbial respiration rates were significantly higher in the undisturbed peat bog sites than in any of the restoration treatments, but the technique used to restore mined peatlands did have a significant effect on these ecosystem process rates. Results suggest that ecosystem processes such as decomposition and microbial community activity recover faster with restoration techniques such as direct transfer of intact habitat islands, than with other techniques such as simple seed addition. However, even after 12 months, litter decomposition and microbial activity in restored habitats were still far from reaching the levels recorded in the undisturbed peat bog. In addition, there was a strong relationship between the effort (and cost) applied to plant community restoration treatments and the rate of decomposition and microbial community activity.






Similar content being viewed by others
References
Atkinson RB, Cairns J (2001) Plant decomposition and litter accumulation in depressional wetlands: functional performance of two wetland age classes that were created via excavation. Wetlands 21:354–362
Barajas-Guzman G, Alvares-Sanchez J (2003) The relationships between litter fauna and rates of litter decomposition in a tropical rain forest. Appl Soil Ecol 24:91–100
Bradford MA, Tordoff GM, Eggers T, Jones TH, Newington JE (2002) Microbiota, fauna and mesh size interactions in litter decomposition. Oikos 99:317–323
Brandenburg A, Sparling GP (1994) A comparison of total C, microbial C and respiration in revegetated, pasture and native woodland habitats in the Western Australian Wheat belt. Soil biota – management in sustainable farming systems, poster papers, CSIRO, Australia, pp 173–175
Bridgham SD, Richardson CJ (1992) Mechanisms controlling soil respiration (CO2 and CH4) in southern peatlands. Soil Biochem 24:1089–1099
Bruelheide B, Flintrop T (2000) Evaluating the transplantation of a meadow in the Hartz Mountains, Germany. Biol Conserv 92:109–120
Clarkson BR (1997) Vegetation recovery following fire in two Waikato peatlands at Whangamarino and Moanatuatua, New Zealand. New Zeal J Bot 35:167–179
Clarkson BR (2002) Swamps, fens and bogs. In: Clarkson B, Merrett M, Downs T (eds) Botany of the Waikato. Waikato Botanical Society Inc., Hamilton, pp 49–58
Clarkson BR, Watts CH (2003) Recovery of vegetation and invertebrates in the restoration of a cut-over peat bog at Torehape, Hauraki Plains. Landcare Research Contract Report: LC0203/170. Landcare Research, Christchurch, New Zealand
Cobbaert D, Rochefort L, Price JS (2004) Experimental restoration of a fen plant community after peat mining. Appl Veget Sci 7:209–220
Cooper DJ, MacDonald LH (2000) Restoring the vegetation of mined peatlands in the Southern Rocky mountains of Colorado, USA. Restor Ecol 8:103–111
Dilly O, Munch JC (2004) Litter decomposition and microbial characteristics in agricultural soils in Northern, Central and Southern Germany. Soil Sci Plant Nutr 50:843–853
Environment Waikato (1998) Waikato state of the environment report. Environment Waikato, Hamilton, New Zealand
Falconer GJ, Wright JW, Beall HW (1933) The decomposition of certain types of fresh litter under field conditions. Am J Bot 20:196–203
Findlay S, Carreiro M, Krischik V, Jones CG (1996) Effects of damage to living plants on leaf litter quality. Ecol Appl 6:269–275
Good JEG, Wallace HL, Stevens PA, Radford GL (1999) Translocation of herb-rich grassland from a site in Wales prior to opencast coal extraction. Restor Ecol 7:337–347
Gorham E, Rochefort L (2003) Peatland restoration: a brief assessment with special reference to Sphagnum bogs. Wetlands Ecol Manage 11:109–119
Harris JA (2003) Measurements of the soil microbial community for estimating the success of restoration. Eur J Soil Sci 54:801–808
Irmler U (2000) Changes in the fauna and its contribution to mass loss and N release during leaf litter decomposition in two deciduous forests. Pedobiologia 44:105–118
Jeffries P, Gianinazzi S, Perotto S, Barea JM (2003) The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fert Soils 37:1–16
Koukoura Z, Mamolos AP, Kalburtji KL (2003) Decomposition of dominant plant species litter in a semi-arid grassland. Appl Soil Ecol 23:13–23
Kurzatkowshki D, Martius C, Hofer H, Garcia M, Forster B, Beck L, Vlek P (2004) Litter decomposition, microbial biomass and activity of soil organisms in three agroforestry sites in Central Amazonia. Nutr Cycling Agroecosyst 69:257–267
Laiho R, Laine J, Trettin CC, Finer L (2004) Scots pine litter decomposition along drainage succession and soil nutrient gradients in peatland forests, and the effects of inter-annual weather variation. Soil Biol Biochem 36:1095–1109
Moynahan OS, Zabinski CA, Gannon JE (2002) Microbial community structure and carbon-utilization diversity in a mine tailings revegetation study. Restor Ecol 10:77–87
Potthoff M, Jackson LE, Steenwerth KL, Ramirez I, Stromberg MR, Rolston DE (2005) Soil biological and chemical properties in restored perennial grassland in California. Restor Ecol 13:1–73
Richert M, Dietrich O, Koppisch D, Roth S (2000) The influence of rewetting on vegetation development and decomposition in a degraded fen. Restor Ecol 8:186–195
Rochefort L, Quinty F, Campeau S, Johnson K, Malterer T (2003) North American approach to the restoration of Sphagnum dominated peatlands. Wetlands Ecol Manage 11:3–20
Sawada Y, Sparling GP, Jasper DA (1994) Use of microbial biomass and activity indices to assess mine-site rehabilitation. In: Conference proceedings of the Australian society of soil science. Soils ’94 Busselton, Western Australia
Schadler M, Brandl R (2005) Do invertebrate decomposers affect the disappearance rate of litter mixtures? Soil Biol Biochem 37:329–337
Schipper L, Clarkson B, Vojvodic-Vukovic M, Webster R (2002) Restoring cut-over restiad peat bogs: a factorial experiment of nutrients, seed and cultivation. Ecol Eng 19:29–40
Smith RS, Shiel RS, Bardgett RD, Millward D, Corkhill P, Rolph G, Peacock S (2003) Soil microbial community, fertility, vegetation and diversity as targets in the restoration management of a meadow grassland. J Appl Ecol 40:51–64
Sundaravalli VM, Paliwal K, Illakiam D (2001) Leaf litter decomposition and soil respiration in a semi-arid ecosystem near Madurai, South India. Int J Ecol Environ Sci 27:221–224
Taylor J, Middleton BA (2004) Comparison of litter decomposition in a natural versus coal-slurry pond reclaimed as a wetland. Land Degrad Dev 15:439–446
Thompson MA, Campbell DJ, Spronken-Smith RA (1999) Evaporation from natural and modified raised peat bogs in New Zealand. Agric Forest Meteorol 95:85–98
Thormann MN, Bayley SE (1997) Decomposition along a moderate-rich fen-marsh peatland gradient in boreal Alberta, Canada. Wetlands 17:123–137
Thormann MN, Bayley SE, Currah RS (2001) Comparison of decomposition of belowground and aboveground plant litters in peatlands of boreal Alberta, Canada. Can J Bot 79:9–22
Topp GC, Ferre PA (2002) The soil solution phase: water content. In: Dane JH, Topp GC (eds) Methods of soil analysis: part 4 – physical methods. Number 5 in the Soil Science Society of America Book Series. Soil Science Society of America Inc., Madison
van Breemen N (1995) How Sphagnum bogs down other plants. Trends Evol Ecol 10:270–275
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability, and productivity. Nature 396:69–72
VSN International (2007) GenStat sixth edition version 8.2.0.235. Lawes Agricultural Trust, Rothamstead
Waddington JM, Rochefort L, Campeau S (2003) Sphagnum production and decomposition in a restored cutover peatland. Wetlands Ecol Manage 11:85–95
Ward SC, Majer JD, O’Connell AM (1991) Decomposition of eucalypt litter on rehabilitated bauxite. Aust J Ecol 6:251–257
Wardle DA (1993) Changes in the microbial biomass and metabolic quotient during leaf litter succession in some New Zealand forest and scrubland ecosystems. Funct Ecol 7:346–355
Watts CH (2006) Invertebrate community reassembly and altered ecosystem process rates following experimental habitat restoration in a mined peat bog in New Zealand. PhD thesis, University of Canterbury, Christchurch, New Zealand
Whinam J, Hope GS, Clarkson BR, Buxton RP, Alspach PA, Adam P (2003) Sphagnum in peatlands of Australasia: their distributions, utilisation and management. Wetlands Ecol Manage 11:37–49
Zedler JB (2000) Progress in wetland restoration ecology. Trends Ecol Evol 15:402–407
Acknowledgements
Research funds were provided by the Foundation for Research, Science and Technology New Zealand (under Contracts C09X0205 and C09X0508). We thank Gamman Mining for kindly providing a study site. We are grateful to the numerous people who assisted with sampling particularly, Neil Fitzgerald, Louise Hunter and Danny Thornburrow. Tim Moore, Louis Schipper, Graham Sparling, William Lee, Anne Austin and two anonymous reviewers provided useful comments on earlier drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendices
Rights and permissions
About this article
Cite this article
Watts, C.H., Vojvodic-Vukovic, M., Arnold, G.C. et al. A comparison of restoration techniques to accelerate recovery of litter decomposition and microbial activity in an experimental peat bog restoration trial. Wetlands Ecol Manage 16, 199–217 (2008). https://doi.org/10.1007/s11273-007-9068-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-007-9068-0