Abstract
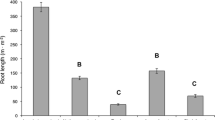
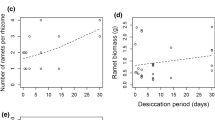
In the last century, Phragmites australis (common reed) has expanded from a minor component of the mid-Atlantic tidal wetlands to a dominant species in many locations. Expansion of Phragmites results in decreased plant diversity and alterations to the tidal characteristics of the marsh, resulting in decreased wetland value. Management efforts have used a variety of strategies in an attempt to control its expansion. We tested a greenhouse bioassay that provided insight into the rhizome vitality of six herbicide-treated sites in the Alloway Creek Watershed, NJ well in advance of the growing season. At three sites, rhizomes were exhumed and classified by depth (0–25 cm and 25–75 cm) and appearance (color and firmness). Concurrently, the same protocol was followed, but conducted on an areal basis at three additional sites. Material was grown in sand under greenhouse conditions void of nutrient supplements for 70 days, after which shoots were removed and the rhizomes replanted for 30 days. Effectiveness of control strategies was quantified by examining rhizome color, vitality, and shoot densities in the field. Color was indicative of quality of rhizome reserves. Less than 0.2% of the firm, brown rhizomes produced shoots upon initial planting and none produced shoots upon replanting, whereas 50.9% of white rhizomes produced shoots on initial planting. Rhizome vitality was quantified by examining shoot emergence and the morphology of the shoots. Coupling rhizome vitality with observed field densities resulted in a predictive capability, and shoot density and biomass predictions were compared to field measurements in July 2001. We tested and accurately predicted the relative shoot densities and shoot biomass of the three sites for which we collected rhizome material on an areal basis. The result is a rapid, valuable, and cost-effective monitoring tool that can quickly quantify the effects of past control methods and predict future growth potential.
Similar content being viewed by others
References
Ailstock MS, Norman CM, Bushmann PJ (2001) Common reed Phragmites australis: control and effects upon biodiversity in freshwater nontidal wetlands. Restor Ecol 9:49–59
Amsberry L, Baker MA, Ewanchuk PJ, Bertness MD (2000) Clonal integration and the expansion of Phragmites australis. Ecol Appl 10:1110–1118
Bertness MD, Ewanchuk PJ, Silliman BR (2002) Anthropogenic modification of New England salt marsh landscapes. Proc Natl Acad Sci USA 99:1395–1398
Chambers RM, Mozdzer TJ, Ambrose JC (1998) Effects of salinity and sulfide on the distribution of Phragmites australis and Spartina alterniflora in a tidal saltmarsh. Aquat Bot 62:161–169
Chambers RM, Meyerson LA, Saltonstall K (1999) Expansion of Phragmites australis into tidal wetlands of North America. Aquat Bot 64:261–273
Chambers RM, Osgood DT, Bart DJ, Montalto F (2003) Phragmites australis invasion and expansion in tidal wetlands: interactions among salinity, sulfide, and hydrology. Estuaries 26:398–406
Gallagher JL (1983) Seasonal patterns in recoverable underground reserves in Spartina alterniflora Loisel. Am J Bot 70:212–215
Gallagher JL, Kibby HV (1981) The streamside effect in a Carex lyngbyei Estuarine Marsh—the possible role of recoverable underground reserves. Estuar Coast Shelf Sci 12:451–460
Hansen RM (1978) Shasta ground sloth food habits, Rampart Cave, Arizona. Paleobiology 4:302–319
Hellings SE, Gallagher JL (1992) The effects of salinity and flooding on Phragmites australis. J Appl Ecol 29:41–49
Keller BEM (2000) Plant diversity in Lythrum, Phragmites, and Typha marshes, Massachusetts, USA. Wetlands Ecol Manage 8:391–401
Kibby HV, Gallagher JL, Sanville WD (1980) Field guide to evaluate net primary production of wetlands. U.S. Environmental Protection Agency, Corvallis, OR
League MT, Seliskar DM, Gallagher JL (2006) Comparison of the rhizome growth dynamics of native and exotic haplotypes of Phragmites austrais (common reed). Estuaries 29(2):in press
Lissner J, Schierup HH (1997) Effects of salinity on the growth of Phragmites australis. Aquat Bot 55:247–260
Minchinton TE, Bertness MD (2003) Disturbance-mediated competition and the spread of Phragmites australis in a coastal marsh. Ecol Appl 13:1400–1416
Moreira I, Monteiro A, Sousa E (1999) Chemical control of common reed (Phragmites australis) by foliar herbicides under different spray conditions. Hydrobiologia 415:299–304
Rooth JE, Stevenson JC, Cornwall JC (2003) Increased sediment accretion rates following invasion by Phragmites australis: the role of litter. Estuaries 26:475–483
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci USA 99:2445–2449
Saltonstall K (2003a) Genetic variation among North American Populations of Phragmites australis: implications for management. Estuaries 26:444–451
Saltonstall K (2003b) Microsatellite variation within and among North American lineages of Phragmites australis. Mol Ecol 12:1689–1702
Saltonstall K (2003c) A rapid method for identifying the origin of North American Phragmites Populations using RFLP analysis. Wetlands 23:1043–1047
Seliskar DM, Gallagher JL, Burdick DM, Mutz LA (2002) The regulation of ecosystem functions by ecotypic variation in the dominant plant: a Spartina alterniflora salt-marsh case study. J Ecol 90:1–11
Seliskar DM, Smart KE, Higashikubo BT, Gallagher JL (2004) Seedling sulfide sensitivity among plant species colonizing Phragmites-infested wetlands. Wetlands 24:426–433
Silliman BR, Bertness MD (2004) Shoreline development drives invasion of Phragmites australis and the loss of plant diversity on New England salt marshes. Conserv Biol 18:1424–1434
Teal JM, Peterson S (2005) The interaction between science and policy in the control of Phragmites in oligohaline marshes of Delaware Bay. Restor Ecol 13:223–227
Teal JM, Weinstein MP (2002) Ecological engineering, design, and construction considerations for marsh restorations in Delaware Bay, USA. Ecol Eng 18:607–618
Turner RE, Warren RS (2003) Valuation of continuous and intermittent Phragmites control. Estuaries 26:618–623
Vasquez EA, Glenn EP, Brown JJ, Guntenspergen GR, Nelson SG (2005) Salt tolerance underlies the cryptic invasion of North American salt marshes by an introduced haplotype of the common reed Phragmites australis (Poaceae). Marine Ecol Prog Ser 298:1–8
Warren RS, Fell PE, Grimsby JL, Buck EL, Rilling GC, Fertik RA (2001) Rates, patterns, and impacts of Phragmites australis expansion and effects of experimental Phragmites control on vegetation, macroinvertebrates, and fish within tidelands of the lower Connecticut River. Estuaries 24:90–107
Weinstein MP, Balletto JH, Teal JM, Ludwig DF (1997) Success criteria and adaptive management for a large-scale wetland restoration project. Wetlands Ecol Manage 4:111–127
Weinstein MP, Teal JM, Balletto JH, Strait KA (2001) Restoration principles emerging from one of the␣worldȁ9s largest tidal marsh restoration projects. Wetlands Ecol Manage 9:387–407
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants in wetlands: opportunities, opportunists, and outcomes. Crit Rev Plant Sci 23:431–452
Acknowledgements
The authors wish to thank Jiangbo Wang, Brenda Evans, Rebecca Rush, Alan Finio, and Joseph Klein for assistance provided. This research was funded by the Estuary Enhancement Program of the Public Service Enterprise Group and the Estuarine Reserves Division, Office of Ocean and Coastal Resource Management, National Ocean Service, National Oceanic and Atmospheric Administration (NOAA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
League, M.T., Seliskar, D.M. & Gallagher, J.L. Predicting the Effectiveness of Phragmites Control Measures using a Rhizome Growth Potential Bioassay. Wetlands Ecol Manage 15, 27–41 (2007). https://doi.org/10.1007/s11273-006-9009-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-006-9009-3