Abstract
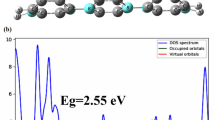
This study aimed to investigate the adsorption characteristic behavior of 4-(((4-(phenyldiazenyl) phenyl) imino) methyl) benzoic acid molecule as an azo dye (AD) on boron nitride nanotube (BNNT) (8,0) by using density functional theory (DFT) method. Thermodynamic function analysis suggests considerable advantages for the adsorption of the mentioned AD onto BNNT. The electronic properties of the AD and BNNT and the combination of them (ADBN) have been explored and discussed. A quantitative approach based on the B3LYP/6–311 + G(d) level of theory investigated dipole moment, adsorption energy, and also the difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), energy gap (HLG). To summarize the electronic structures, the total density of states (DOS) were plotted. To predict the compound’s reactivity, chemical hardness (η), electronic chemical potential (µ), and electrophilicity index (ω) were studied. The highest and lowest asymmetric charge distributions on azo dye and boron nitride nanotube (BNNT) compound, which lead to the dipole moments complex, were verified by contour maps. Therefore, AD may be taken into consideration as a strong electrophile. The adsorption behavior of AD on aluminum-phosphide nanotube (8,0) (AlPNT) and the combination of them (ADAlP) by using DFT method have been investigated and discussed to comparison.






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Ahmadi, A., Hadipour, N. L., Kamfiroozi, M., & Bagheri, Z. (2012). Theoretical study of aluminum nitride nanotubes for chemical sensing of formaldehyde. Sensors and Actuators B: Chemical, 161, 1025–1029. https://doi.org/10.1016/j.snb.2011.12.001
Attar, H. M., & Rezaei, R. (2006). Investigating the efficiency of advanced photochemical oxidation (APO) technology in degradation of direct azo dye by UV/H2O2 Process. Water and Wastewater, 17–3(59), 75–83.
Balasubramanian, C., Bellucci, S., Castrucci, P., De Crescenzi, M., & Bhoraskar, S. V. (2004). Scanning tunneling microscopy observation of coiled aluminum nitride nanotubes. Chemical Physics Letters, 383, 188–191. https://doi.org/10.1016/j.cplett.2003.11.028
Beck, A. D. (1993). Density-functional thermochemistry III The role of exact exchange. The Journal of Chemical Physics, 98, 5648–5656.
Bredas, J. L. (2014). Mind the gap! Mater. Horizons., 1, 17–19.
Chattaraj, P.K., Giri, S., Duley, S. (2011) Update 2 of: Electrophilicity index. Chemical Reviews 111, PR43-PR75. https://doi.org/10.1021/cr100149p.
Chen, X., Ma, J., Hu, Z., Wu, Q., & Chen, Y. (2005). AIN nanotube: Round or faceted? Journal of the American Chemical Society, 127, 7982–7983. https://doi.org/10.1021/ja051505y
Chen, X., Wu, P., Rousseas, M., Okawa, D., Gartner, Z., Zettl, A., & Bertozzi, C. R. (2009). Boron nitride nanotubes are noncytotoxic and can be functionalized for interaction with proteins and cells. Journal of the American Chemical Society, 131, 890–891. https://doi.org/10.1021/ja807334b
Chopra, N. G., Luyken, R. J., Cherrey, K., Crespi, V. H., Cohen, M. L., Louie, S. G., & Zettl, A. (1995). Boron nitride nanotubes. Science, 269, 966–967. 80-.
Dastgerdi, Z. H., Meshkat, S. S., & Esrafili, M. D. (2019). Enhanced adsorptive removal of Indigo carmine dye performance by functionalized carbon nanotubes based adsorbents from aqueous solution: Equilibrium, kinetic, and DFT study. Journal of Nanostructure in Chemistry, 9, 323–334. https://doi.org/10.1007/s40097-019-00321-0
De Almeida, E. F., De Brito Mota, F., De Castilho, C. M. C., Kakanakova-Georgieva, A., & Gueorguiev, G. K. (2012). Defects in hexagonal-AIN sheets by first-principles calculations. The European Physical Journal B, 85, 1–9. https://doi.org/10.1140/epjb/e2011-20538-6
Domingo, L. R., Aurell, M. J., Pérez, P., & Contreras, R. (2002). Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron, 58, 4417–4423. https://doi.org/10.1016/S0040-4020(02)00410-6
Farghali, A. A., Bahgat, M., ElRouby, W. M. A., & Khedr, M. H. (2013). Decoration of multi-walled carbon nanotubes (MWCNTs) with different ferrite nanoparticles and its use as an adsorbent. Journal of Nanostructure in Chemistry, 3, 1–12. https://doi.org/10.1186/2193-8865-3-50
Feller, D. (1996). The role of databases in support of computational chemistry calculations. Journal of Computational Chemistry, 17, 1571–1586. https://doi.org/10.1002/(SICI)1096-987X(199610)17:13%3c1571::AID-JCC9%3e3.0.CO;2-P
Foster, J. P., & Weinhold, F. (1980). Natural hybrid orbitals. Journal of the American Chemical Society, 102, 7211–7218. https://doi.org/10.1021/ja00544a007
Frisch, M., Trucks,G. W., Schlegel, H. B., Scuseria, G. E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A. (2009). Gaussian 09, revision D. 01.
Glendening, E.D., Reed, A.E., Carpenter, J.E., Weinhold, F. (n.d.). NBO 3.0 program manual, Theor. Chem. Institute, Univ. W.
Goldberger, J., He, R., Zhang, Y., Lee, S., Yan, H., Choi, H. J., & Yang, P. (2003). Single-crystal gallium nitride nanotubes. Nature, 422, 599–602. https://doi.org/10.1038/nature01551
Gonzalez-Ortiz, D., Salameh, C., Bechelany, M., & Miele, P. (2020). Nanostructured boron nitride–based materials: Synthesis and applications. Materials Today Advances, 8, 100107.
Grimme, S. (2006). Semiempirical GGA-type density functional constructed with a long-range dispersion correction. Journal of Computational Chemistry, 27, 1787–1799. https://doi.org/10.1002/jcc.20495
Hao, C., Li, G., Wang, G., Chen, W., & Wang, Sh. (2022). Preparation of acrylic acid modified alkalized MXene adsorbent and study on its dye adsorption performance. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 632, 127730. https://doi.org/10.1016/j.colsurfa.2021.127730
Hoffmann, R., Schleyer, P. V. R., & Schaefer, H. F., III. (2008). Predicting molecules—More realism, please. Angewandte Chemie International Edition, 47, 7164–7167.
Iijima, S. (1991). Helical microtubules of graphitic carbon. Nature, 354, 56–58. https://doi.org/10.1038/354056a0
Jensen, F. (2007). Introduction to computational chemistry. John Wiley & Sons. https://doi.org/10.1007/s00214-013-1372-6
Jha, N., & Ramaprabhu, S. (2009). Thermal conductivity studies of metal dispersed multiwalled carbon nanotubes in water and ethylene glycol based nanofluids. Journal of Applied Physics, 106, 84317. https://doi.org/10.1063/1.3240307
Kang, Y. J., Hong, H. M., Moon, C. Y., Chang, K. J., & Kim, H. J. (2006). Stability and electronic structure of aluminate nanotubes. Journal of the Korean Physical Society, 48, 1351–1354.
Koopmans, T. (1933). Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica, 1, 104–113. http://ci.nii.ac.jp/naid/10010180566/en/
Kumar, A., Shalini, G., Sharma, M., Naushad, A., Kumar, S., Kalia, C., & Guo, G. T. (2017). Mola, Facile hetero-assembly of superparamagnetic Fe3O4/BiVO4 stacked on biochar for solar photo-degradation of methyl paraben and pesticide removal from soil. Journal of Photochemistry and Photobiology A: Chemistry, 337, 118–131. https://doi.org/10.1016/j.jphotochem.2017.01.010
Kumar, A., Rana, A., Sharma, G., Naushad, M., Dhiman, P., Kumari, A., & Stadler, F. J. (2019). Recent advances in nano-Fenton catalytic degradation of emerging pharmaceutical contaminants. Journal of Molecular Liquids, 290, 111177. https://doi.org/10.1016/j.molliq.2019.111177
Levine, I. N. (1995). Multi component phase equilibrium. Physical Chemistry, 6, 352–356.
Li, Y. H., Zhu, Y., Zhao, Y., Wu, D., & Luan, Z. (2006). Different morphologies of carbon nanotubes effect on the lead removal from aqueous solution. Diamond and Related Materials, 15, 90–94. https://doi.org/10.1016/j.diamond.2005.07.004
Lopes, A., Martins, S., Morão, A., Magrinho, M., & Gonçalves, I. (2004). Degradation of a textile dye C I Direct Red 80 by electrochemical processes. Portugaliae Electrochimica Acta, 22, 279–294. https://doi.org/10.4152/pea.200403279
Lu, C., & Chiu, H. (2006). Adsorption of zinc(II) from water with purified carbon nanotubes. Chemical Engineering Science, 61, 1138–1145. https://doi.org/10.1016/j.ces.2005.08.007
Lu, C., Chiu, H., & Liu, C. (2006). Removal of zinc(II) from aqueous solution by purified carbon nanotubes: Kinetics and equilibrium studies. Industrial and Engineering Chemistry Research, 45, 2850–2855. https://doi.org/10.1021/ie051206h
Ma, X., Li, Z., Achenie, L. E. K., & Xin, H. (2015). Machine-learning-augmented chemisorption model for CO2 electroreduction catalyst screening. Journal of Physical Chemistry Letters, 6, 3528–3533.
Majlesi, K., Zare, K., & Teimouri, F. (2004). Dependence on ionic strength of formation constants, protonation, and complexation of nitrilotriacetic acid with tungsten(VI) in sodium perchlorate aqueous solution. Journal of Chemical and Engineering Data, 49, 439–443. https://doi.org/10.1021/je034092u
Mirzaei, M., & Mirzaei, M. (2010). A computational study of oxygen-termination of a (6, 0) boron nitride nanotube. Monatshefte für Chemie - Chemical Monthly, 141, 491–494.
Mittal, A., Mittal, J., & Kurup, L. (2006). Adsorption isotherms, kinetics and column operations for the removal of hazardous dye, Tartrazine from aqueous solutions using waste materials—Bottom Ash and De-Oiled Soya, as adsorbents. Journal of Hazardous Materials, 136, 567–578.
Moradi, O., Shahryari-Ghoshekandi, R., Sadegh, H., Norouzi, M. (2016). Application of carbon nanotubes in nanomedicine: New medical approach for tomorrow, In: Med. Imaging Concepts, Methodol. Tools, Appl., IGI Global, 2021–2062. https://doi.org/10.4018/978-1-5225-0571-6.ch082
Musa, A. Y., Kadhum, A. A. H., Mohamad, A. B., Rahoma, A. A. B., & Mesmari, H. (2010). Electrochemical and quantum chemical calculations on 4, 4-dimethyloxazolidine-2-thione as inhibitor for mild steel corrosion in hydrochloric acid. Journal of Molecular Structure, 969, 233–237.
Nørskov, J. K., Bligaard, T., Rossmeisl, J., & Christensen, C. H. (2009). Towards the computational design of solid catalysts. Nature Chemistry, 1, 37–46.
O’Boyle, N. M., Tenderholt, A. L., & Langner, K. M. (2008). Cclib: A library for package-independent computational chemistry algorithms. Journal of Computational Chemistry, 29, 839–845. https://doi.org/10.1002/jcc.20823
Park, Y.-G., Nam, S.-N., Jang, M., Park, C. M., Her, N., Sohn, J., Cho, J., & Yoon, Y. (2022). Boron nitride-based nanomaterials as adsorbents in water: A review. Separation and Purification Technology, 288, 120637.
Parr, R. G., Szentpály, L. V., & Liu, S. (1999). Electrophilicity index. Journal of the American Chemical Society, 121, 1922–1924.
Peng, X., Luan, Z., Di, Z., Zhang, Z., & Zhu, C. (2005). Carbon nanotubes-iron oxides magnetic composites as adsorbent for removal of Pb(II) and Cu(II) from water. Carbon, 43, 880–883. https://doi.org/10.1016/j.carbon.2004.11.009
Qian, Z., Hou, S., Zhang, J., Li, R., Shen, Z., Zhao, X., & Xue, Z. (2005). Stability and electronic structure of single-walled InN nanotubes. Physica E: Low-dimensional Systems and Nanostructures, 30, 81–85. https://doi.org/10.1016/j.physe.2005.07.002
Rahmanivahid, B., Naderi, F., & Nayebzadeh, H. (2020). Removing methyl orange molecules from aqueous medium by magnetic nanoparticles: Evaluating adsorption factors, isotherms, kinetics and thermodynamics. Journal of Water and Environmental Nanotechnology, 5, 1–16. https://doi.org/10.22090/jwent.2020.01.001
Rakhi, R. B., Sethupathi, K., & Ramaprabhu, S. (2008). Field emission from carbon nanotubes on a graphitized carbon fabric. Carbon, 46, 1656–1663. https://doi.org/10.1016/j.carbon.2008.07.024
Reddy, A. L. M., & Ramaprabhu, S. (2007). Nanocrystalline metal oxides dispersed multiwalled carbon nanotubes as supercapacitor electrodes. Journal of Physical Chemistry C, 111, 7727–7734. https://doi.org/10.1021/jp069006m
Reed, A. E., Curtiss, L. A., & Weinhold, F. (1988). Intermolecular interactions from a natural bond orbital, donor—acceptor viewpoint. Chemical Reviews, 88, 899–926. https://doi.org/10.1021/cr00088a005
Rezaei-Sameti, M. (2010). DFT study on influence of Si and Ge doping on the chemical shielding tensors in the armchair AlN nanotubes. Physica E: Low-dimensional Systems and Nanostructures, 43, 588–592. https://doi.org/10.1016/j.physe.2010.09.016
Robinson, T., McMullan, G., Marchant, R., & Nigam, P. (2001). Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresource Technology, 77, 247–255. https://doi.org/10.1016/S0960-8524(00)00080-8
Rubio, A., Corkill, J. L., & Cohen, M. L. (1994). Theory of graphitic boron nitride nanotubes. Physical Review B, 49, 5081–5084. https://doi.org/10.1103/PhysRevB.49.5081
Samadi, A., Wang, Z., Wang, Sh., Nataraj, S. K., Kong, L., & Zhao, Sh. (2023). Polyaniline-based adsorbents for water treatment: Roles of low-cost materials and 2D materials. Chemical Engineering Journal, 478, 147506. https://doi.org/10.1016/j.cej.2023.147506
Sharifi, M., Marjani, A., Mahdavian, L., & Shamlouei, H. R. (2022). Density functional theory study of dyes removal from colored wastewater by a nano-composite of polysulfone/polyethylene glycol. Journal of Nanostructure in Chemistry, 1–14. https://doi.org/10.1007/s40097-022-00502-4
Sheikhi, M., Shahab, S., Filippovich, L., Khaleghian, M., Dikusar, E., & Mashayekhi, M. (2017). Interaction between new synthesized derivative of (E, E)-azomethines and BN (6, 6–7) nanotube for medical applications: Geometry optimization, molecular structure, spectroscopic (NMR, UV/Vis, excited state), FMO, MEP and HOMO-LUMO investigations. Journal of Molecular Structure, 1146, 881–888.
Song, W., Ni, M., Lu, J., Gao, Z., Nagase, S., Yu, D., Ye, H., & Zhang, X. (2005). Encapsulations of La@C82 and La2@C80 inside single-walled boron nitride nanotubes. Journal of Molecular Structure: THEOCHEM, 730, 121–124. https://doi.org/10.1016/j.theochem.2005.06.016
Stafiej, A., & Pyrzynska, K. (2007). Adsorption of heavy metal ions with carbon nanotubes. Separation and Purification Technology, 58, 49–52. https://doi.org/10.1016/j.seppur.2007.07.008
Tuzen, M., & Soylak, M. (2007). Multiwalled carbon nanotubes for speciation of chromium in environmental samples. Journal of Hazardous Materials, 147, 219–225. https://doi.org/10.1016/j.jhazmat.2006.12.069
Van de Voorde, M., Werner, M., Fecht, H.-J. (2015) Nano-micro interface, Wiley Online Library.
Vurgaftman, I., & Meyer, J. R. (2003). Band parameters for nitrogen-containing semiconductors. Journal of Applied Physics, 94, 3675–3696. https://doi.org/10.1063/1.1600519
Weinhold, F., & Landis, C. R. (2001). Natural bond orbitals and extensions of localized bonding concepts. Chemistry Education Research and Practice, 2, 91–104. https://doi.org/10.1039/b1rp90011k
Weinhold, F., & Landis, C. R. (2012). Discovering chemistry with natural bond orbitals. John Wiley & Sons. https://doi.org/10.1002/9781118229101
Yang, W., Ding, P., Zhou, L., Yu, J., Chen, X., & Jiao, F. (2013). Preparation of diamine modified mesoporous silica on multi-walled carbon nanotubes for the adsorption of heavy metals in aqueous solution. Applied Surface Science, 282, 38–45. https://doi.org/10.1016/j.apsusc.2013.05.028
Yeo, B. C., Kim, D., Kim, C., & Han, S. S. (2019). Pattern learning electronic density of states. Science and Reports, 9, 5879.
Yim, W. M., Stofko, E. J., Zanzucchi, P. J., Pankove, J. I., Ettenberg, M., & Gilbert, S. L. (1973). Epitaxially grown AlN and its optical band gap. Journal of Applied Physics, 44, 292–296. https://doi.org/10.1063/1.1661876
Yu, J., Chen, Y., & Cheng, B. M. (2009). Dispersion of boron nitride nanotubes in aqueous solution with the help of ionic surfactants. Solid State Communications, 149, 763–766. https://doi.org/10.1016/j.ssc.2009.03.001
Zare, K., Gupta, V. K., Moradi, O., Makhlouf, A. S. H., Sillanpää, M., Nadagouda, M. N., Sadegh, H., Shahryari-ghoshekandi, R., Pal, A., Wang, Z., Tyagi, I., & Kazemi, M. (2015). A comparative study on the basis of adsorption capacity between CNTs and activated carbon as adsorbents for removal of noxious synthetic dyes: A review. Journal of nanostructure in chemistry, 5, 227–236. https://doi.org/10.1007/s40097-015-0158-x
Zhang, D., & Zhang, R. Q. (2003). Theoretical prediction on aluminum nitride nanotubes. Chemical Physics Letters, 371, 426–432. https://doi.org/10.1016/S0009-2614(03)00289-6
Zhao, M., Xia, Y., Tan, Z., Liu, X., Li, F., Huang, B., Ji, Y., & Mei, L. (2004). Strain energy and thermal stability of single-walled aluminum nitride nanotubes from first-principles calculations. Chemical Physics Letters, 389, 160–164. https://doi.org/10.1016/j.cplett.2004.03.082
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gharebiglu, T., Naderi, F., Heydarinasab, A. et al. Density Functional Theory Study of Removal of 4-(((4-(Phenyldiazenyl) Phenyl) Imino) Methyl) Benzoic Acid Azo Dye by Boron Nitride-Nanotube, to Investigate a Model for Industrial Wastewater Treatment. Water Air Soil Pollut 235, 112 (2024). https://doi.org/10.1007/s11270-024-06902-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-06902-2