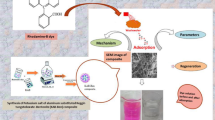
Abstract
Many dyeing industries release toxic effluents to water bodies that can severely affect human health, aquatic life, and other living organisms. Hence, the treatment of dyeing effluent is mandatory to protect the environment. Bentonite (BT) clay and hydroxyapatite (HA) ceramic material possess appreciable dye adsorption capacity. But BT and HA cause pressure drops during filtration due to their powder form. To overcome this problem and to enhance the dye adsorption capacity of BT and HA, the present study concentrates on the development of a hybrid clay-ceramic composite using chitosan (CS) biopolymer namely BTHACS composite. The developed BTHACS composite was employed for methylene blue (MB) removal. The highest dye adsorption capacity of 45.5 mg/g was obtained for BTHACS composite within 40 min contact time than the individual components (BT, HA, and CS). Various adsorption parameters, namely, initial MB concentration, contact time, pH, dosage, interfering ions, and temperature studies were performed under batch method. The nature of BTHACS composite and its MB adsorption were analyzed by FTIR, EDAX, and SEM analysis. The isotherm and kinetic studies were also investigated with different initial concentrations (80, 100, 120, and 140 mg/L) at 303, 313, and 323 K. Thermodynamic studies results confirm that the MB adsorption onto BTHACS composite was spontaneous, feasible, and endothermic in nature. The regeneration studies indicate that BTHACS composite has high reusable ability, and it can be recyclable upto 5 cycles. The field suitability results provide that BTHACS composite has a high potential for MB removal.






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Aaddouz, M., et al. (2023). Removal of methylene blue from aqueous solution by adsorption onto hydroxyapatite nanoparticles. Journal of Molecular Structure, 1288, 135807.
Abdessemed, D., Nezzal, G., & Aim, R. B. (2000). Coagulation—adsorption—ultrafiltration for wastewater treatment and reuse. Desalination, 131(1–3), 307–314.
Abutaleb, A., et al. (2023). Fe3O4-multiwalled carbon nanotubes-bentonite as adsorbent for removal of methylene blue from aqueous solutions. Chemosphere, 316, 137824.
Ahmad, R., & Ejaz, M. O. (2022). Adsorption of methylene blue dye from aqueous solution onto synthesized bentonite/silvernanoparticles-alginate (Bent/AgNPs-Alg) bio-nanocomposite. Biomass Conversion and Biorefinery, 1–16. https://doi.org/10.1007/s13399-022-03350-y
Ai, L., & Jiang, J. (2012). Removal of methylene blue from aqueous solution with self-assembled cylindrical graphene–carbon nanotube hybrid. Chemical Engineering Journal, 192, 156–163.
Al-Mamun, M. R., et al. (2019). Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. Journal of Environmental Chemical Engineering, 7(5), 103248.
Almeida, C. A., et al. (2009). Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. Journal of Colloïd and Interface Science, 332(1), 46–53.
Annadurai, G., Juang, R. S., & Lee, D. J. (2002). Factorial design analysis for adsorption of dye on activated carbon beads incorporated with calcium alginate. Advances in Environmental Research, 6(2), 191–198.
APHA. (2005). Standard methods for the examination of water and wastewater. American Public Health Association.
Benhouria, A., Islam, M. A., Zaghouane-Boudiaf, H., Boutahala, M., & Hameed, B. H. (2015). Calcium alginate–bentonite–activated carbon composite beads as highly effective adsorbent for methylene blue. Chemical Engineering Journal, 270, 621–30.
Dai, H., Huang, Y., & Huang, H. (2018). Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue. Carbohydrate Polymers, 185, 1–1.
Deng, Y., & Zhao, R. (2015). Advanced oxidation processes (AOPs) in wastewater treatment. Current Pollution Reports, 1, 167–176.
Dotto, G. L., et al. (2016). Development of chitosan/bentonite hybrid composite to remove hazardous anionic and cationic dyes from colored effluents. Journal of Environmental Chemical Engineering, 4(3), 3230–3239.
Du, P. Y., Gu, W., & Liu, X. (2016). Multifunctional three-dimensional europium metal–organic framework for luminescence sensing of benzaldehyde and Cu2+ and selective capture of dye molecules. Inorganic Chemistry, 55(16), 7826–8.
Eltaweil, A. S., et al. (2022). Graphene oxide incorporated cellulose acetate beads for efficient removal of methylene blue dye; isotherms, kinetic, mechanism and co-existing ions studies. Journal of Porous Materials, 30(2), 1–12.
Freundlich, H. M. F. (1906). Over the adsorption in solution. Journal of Physical Chemistry, 57(385471), 1100–1107.
Graaf, G. H., et al. (1990). Intra-particle diffusion limitations in low-pressure methanol synthesis. Chemical Engineering Science, 45(4), 773–783.
Guesmi, Y., et al. (2018). Synthesis of hydroxyapatite-sodium alginate via a co-precipitation technique for efficient adsorption of Methylene Blue dye. Journal of Molecular Liquids, 249, 912–920.
Hamad, H. N., & Idrus, S. (2022). Recent developments in the application of bio-waste-derived adsorbents for the removal of methylene blue from wastewater: A review. Polymers, 14(4), 783.
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34(5), 451–465.
Hutson, N. D., & Yang, R. T. (1997). Theoretical basis for the Dubinin-Radushkevitch (DR) adsorption isotherm equation. Adsorption, 3, 189–195.
Jebli, A., et al. (2023). Synthesis of a chitosan@ hydroxyapatite composite hybrid using a new approach for high-performance removal of crystal violet dye in aqueous solution, equilibrium isotherms and process optimization. Journal of the Taiwan Institute of Chemical Engineers, 149, 105006.
Jeyaseelan, A., et al. (2023). Micro-encapsulation of rare earth metal ion-doped magnesia-based alginate/pectin hybrid polymeric composites for defluoridation of water. Reaction Chemistry & Engineering, 8, 3171–3184.
Katti, K. S., Katti, D. R., & Dash, R. (2008). Synthesis and characterization of a novel chitosan/montmorillonite/hydroxyapatite nanocomposite for bone tissue engineering. Biomedical Materials, 3(3), 034122.
Khan, A. A., & Singh, R. P. (1987). Adsorption thermodynamics of carbofuran on Sn (IV) arsenosilicate in H+, Na+ and Ca2+ forms. Colloids and Surfaces, 24(1), 33–42.
Khan, I., et al. (2022). Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water, 14(2), 242.
Kramers, H. A. (1940). Brownian motion in a field of force and the diffusion model of chemical reactions. Physica, 7(4), 284–304.
Kubra, K. T., Salman, M. S., & Hasan, M. N. (2021). Enhanced toxic dye removal from wastewater using biodegradable polymeric natural adsorbent. Journal of Molecular Liquids, 328, 115468.
Kumar, A., & Lingfa, P. (2020). Sodium bentonite and kaolin clays: Comparative study on their FT-IR, XRF, and XRD. Materials Today: Proceedings, 22, 737–742.
Langmuir, I. (1916). The constitution and fundamental properties of solids and liquids. Part I. Solids. Journal of the American chemical society, 38(11), 2221–2295.
Li, W., et al. (2018). Facile fabrication of gelatin/bentonite composite beads for tunable removal of anionic and cationic dyes. Chemical Engineering Research and Design, 134, 336–346.
Mahdavinia, G. R., et al. (2017). Novel magnetic polyvinyl alcohol/laponite RD nanocomposite hydrogels for efficient removal of methylene blue. Journal of Environmental Chemical Engineering, 5(3), 2617–2630.
Mahmoud, G. A., et al. (2020). Chitosan biopolymer based nanocomposite hydrogels for removal of methylene blue dye. SN Applied Sciences, 2, 1.
Mobasherpour, I., et al. (2007). Synthesis of nanocrystalline hydroxyapatite by using precipitation method. Journal of Alloys and Compounds, 430(1–2), 330–333.
Munnaf, A., et al. (2014). Investigation of water quality parameters discharged from textile dyeing industries. Journal of Environmental Science and Natural Resources, 7(1), 257–263.
Mustafa, I. (2019). Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchemical Journal, 144, 397–402.
Nawaz, M. S., & Ahsan, M. (2014). Comparison of physico-chemical, advanced oxidation and biological techniques for the textile wastewater treatment. Alexandria Engineering Journal, 53(3), 717–722.
Pandey, L. M. (2019). Enhanced adsorption capacity of designed bentonite and alginate beads for the effective removal of methylene blue. Applied Clay Science, 169, 102–111.
Pereira, M. B., et al. (2020). Modulating the structure of organofunctionalized hydroxyapatite/tripolyphosphate/chitosan spheres for dye removal. Journal of Environmental Chemical Engineering, 8(4), 103980.
Pleshko, N., Boskey, A., & Mendelsohn, R. (1991). Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophysical Journal, 60(4), 786–793.
Rashid, R., et al. (2021). A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environmental Science and Pollution Research, 28, 9050–9066.
Rinaudo, M. (2006). Chitin and chitosan: Properties and applications. Progress in Polymer Science, 1(7), 603–632.
Salhab, M., Al Sarakbi, W., & Mokbel, K. (2005). Skin and fat necrosis of the breast following methylene blue dye injection for sentinel node biopsy in a patient with breast cancer. International Seminars in Surgical Oncology, 2, 1–3. BioMed Central.
Shafiq, F., et al. (2023). Adsorption mechanism and synthesis of adjustable hollow hydroxyapatite spheres for efficient wastewater cationic dyes adsorption. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 672, 131713.
Shi, L., et al. (2023). Adsorption of methylene blue from aqueous solution by crosslinked carboxymethyl cellulose/organo-montmorillonite composite hydrogels. Journal of Polymer Research, 30(8), 305.
Shindhal, T., et al. (2021). A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered, 12(1), 70–87.
Ullah, N., et al. (2022). Synthesis of activated carbon-surfactant modified montmorillonite clay-alginate composite membrane for methylene blue adsorption. Chemosphere, 309, 136623.
Ulu, A., et al. (2022). Eco-friendly chitosan/κ-carrageenan membranes reinforced with activated bentonite for adsorption of methylene blue. Materials Chemistry and Physics, 278, 125611.
Vigneshwaran, S., et al. (2021). In situ fabrication of ternary TiO2 doped grafted chitosan/hydroxyapatite nanocomposite with improved catalytic performance for the removal of organic dyes: Experimental and systemic studies. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 611, 125789.
Wei, W., et al. (2015). Fast removal of methylene blue from aqueous solution by adsorption onto poorly crystalline hydroxyapatite nanoparticles. Digest Journal of Nanomaterials and Biostructures, 19, 1343–1363.
Wong, Y. C., et al. (2004). Pseudo-first-order kinetic studies of the sorption of acid dyes onto chitosan. Journal of Applied Polymer Science, 92(3), 1633–1645.
Yan, M., Huang, W., & Li, Z. (2019). Chitosan cross-linked graphene oxide/lignosulfonate composite aerogel for enhanced adsorption of methylene blue in water. International Journal of Biological Macromolecules, 136, 927–935.
Zhang, W., et al. (2011). Fast and considerable adsorption of methylene blue dye onto graphene oxide. Bulletin of Environmental Contamination and Toxicology, 87, 86–90.
Acknowledgements
The corresponding author (Dr. N. Viswanathan) dedicated his 100th Science Citation Index (SCI) research article to his beloved father Mr. N. Natrayasamy for his constant encouragement and moral support. The authors thank the researchers supporting project number (RSP2023R169), King Saud University, Riyadh, Saudi Arabia, for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sathiyajothi, V., Viswanathan, N., Kumar, I.A. et al. Development and Characterization of Bentonite/Hydroxyapatite-Based Chitosan Hybrid Composite for Effective Removal of Methylene Blue. Water Air Soil Pollut 234, 766 (2023). https://doi.org/10.1007/s11270-023-06769-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06769-9