Abstract
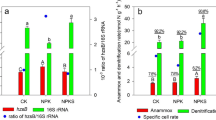
Arsenite (As(III)) oxidation coupled with nitrate (NO3−) reduction is thermodynamically favored and experimentally tested. However, little is known about the functional bacteria responsible for this coupling process in flooded paddy soil. In this study, three microcosms containing paddy soil amended with 1) As(III) + NO3−, 2) As(III), and 3) NO3− were set up to investigate the coupled processes of As(III) oxidation and NO3− reduction and to characterize the associated functional bacteria. The results showed that As(III) was completely oxidized in the Soil + As(III) + NO3− treatment, while no As(III) oxidation was observed with As(III) amendment. NO3− reduction was observed in both the Soil + As(III) + NO3− and Soil + NO3− treatments, where nitrite (NO2−) was the major product. In the Soil + As(III) + NO3− treatment, the 16S rRNA-based dominant genera were Vogesella, Dechloromonas, and Pseudogulbenkiania; the aoxB-based dominant arsenite-oxidizers included Cupriavidus and Acidovorax; and the narG-based dominant nitrate reducers (NO3− → NO2−) were Dechloromonas and Pseudogulbenkiania. Comparison of the aoxB and narG gene markers at 90% similarity indicated a higher diversity of aoxB than narG gene markers based on α-diversity indices in the As(III) + NO3− treatment. In addition, although the dominant arsenite-oxidizing bacteria Cupriavidus and Acidovorax had a lower relative abundance in paddy soil, they played an important role in As(III) oxidation. The findings of this study provide a better understanding of the functional microbial communities involved in As(III) oxidation coupled with NO3− reduction in anoxic paddy soil.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are not openly available and are available from the corresponding author upon reasonable request.
References
Achenbach, L. A., Michaelidou, U., Bruce, R. A., Fryman, J., & Coates, J. D. (2001). Dechloromonas agitata gen. nov., sp. nov. and Dechlorosoma suillum gen. nov., sp. nov., two novel environmentally dominant (per)chlorate-reducing bacteria and their phylogenetic position. International Journal of Systematic and Evolutionary Microbiology, 51, 527–533.
Afkar, E., Lisak, J., Saltikov, C., Basu, P., Oremland, R. S., & Stolz, J. F. (2003). The respiratory arsenate reductase from Bacillus selenitireducens strain MLS10. FEMS Microbiology Letters, 226, 107–112.
Bruce, R. A., Achenbach, L. A., & Coates, J. D. (1999). Reduction of (per)chlorate by a novel organism isolated from paper mill waste. Environmental Microbiology, 1, 319–329.
Byrne-bailey, K. G., Weber, K. A., & Coates, J. D. (2012). Draft genome sequence of the anaerobic, nitrate-dependent, Fe(II)-oxidizing bacterium Pseudogulbenkiania ferrooxidans strain 2002. Journal of Bacteriology, 194, 2400–2401.
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., Fierer, N., Pea, A. G., Goodrich, J. K., & Gordon, J. I. (2010). QIIME allows analysis of high-throughput community sequencing data. Nature Methods, 7, 335–336.
Chakraborty, A., & Picardal, F. (2013). Neutrophilic, nitrate-dependent, Fe(II) oxidation by a Dechloromonas species. World Journal of Microbiology & BiotechnoloGy, 29, 617–623.
Coates, J. D., Chakraborty, R., Lack, J. G., O’Connor, S. M., Cole, K. A., Bender, K. S., & Achenbach, L. A. (2001). Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of Dechloromonas. Nature, 411, 1039–1043.
Coelho, C., González, P. J., & Moura, J. J. G. (2011). The crystal structure of Cupriavidus necator nitrate reductase in oxidized and partially reduced states. Journal of Molecular Biology, 408, 932–948.
Cole, J. R., Chai, B., Marsh, T. L., Farris, R. J., Wang, Q., Kulam, S. A., Chandra, S., Mcgarrell, D. M., Schmidt, T. M., & Garrity, G. M. (2003). The ribosomal database project (RDP-II): Previewing a new auto aligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Research, 31, 442–443.
Ding, L. J., Su, J. Q., Xu, H. J., Jia, Z. J., & Zhu, Y. G. (2015). Long-term nitrogen fertilization of paddy soil shifts iron-reducing microbial community revealed by RNA-13C-acetate probing coupled with pyrosequencing. The ISME Journal, 9, 721–734.
Dowdle, P. R., Laverman, A. M., & Oremland, R. S. (1996). Bacterial dissimilatory reduction of arsenic(V) to arsenic(III) in anoxic sediments. Applied and Environmental Microbiology, 62, 1664–1669.
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics, 26, 2460–2461.
Fodor, P. (2001). Arsenic speciation in the environment. Trace Element Speciation for Environment, Food and Health, Chapter, 11, 196–210.
Gibney, B. P., & Nuesslein, K. (2007). Arsenic sequestration by nitrate respiring microbial communities in urban lake sediments. Chemosphere, 70, 329–336.
Glaser, P., Danchin, A., Kunst, F., Zuber, P., & Nakano, M. M. (1995). Identification and isolation of a gene required for nitrate assimilation and anaerobic growth of Bacillus subtilis. Journal of Bacteriology, 177, 1112–1115.
Grimes, D. J., Woese, C. R., Macdonell, M. T., & Colwell, R. R. (1997). Systematic study of the genus Vogesella gen. nov. and its type species, Vogesella indigofera comb. nov. International Journal of Systematic Bacteriology, 47, 19–27.
Heyrman, J., Vanparys, B., Logan, N. A., Balcaen, A., Rodríguez-Díaz, M., Felske, A., & De Vos, P. (2004). Bacillus novalis sp. nov., Bacillus vireti sp. nov., Bacillus soli sp. nov., Bacillus bataviensis sp. nov. and Bacillus drentensis sp. nov., from the Drentse A grasslands. International journal of systematic and evolutionary microbiology, 54, 47–57.
Higashioka, Y., Kojima, H., Watanabe, M., & Fukui, M. (2013). Desulfatitalea tepidiphila gen. nov., sp. nov., a sulfate-reducing bacterium isolated from tidal flat sediment. International Journal of Systematic & Evolutionary Microbiology, 63, 761–765.
Hoeft, S. E. (2007). Alkalilimnicola ehrlichii sp. nov., a novel, arsenite-oxidizing haloalkaliphilic gammaproteobacterium capable of chemoautotrophic or heterotrophic growth with nitrate or oxygen as the electron acceptor. International Journal of Systematic & Evolutionary Microbiology, 57, 504–512.
Hong, K. W., Thinagaran, D. A., Gan, H. M., Yin, W. F., & Chan, K. G. (2012). Whole-genome sequence of Cupriavidus sp. strain BIS7, a heavy-metal-resistant bacterium. Journal of Bacteriology, 194, 6324–6324.
Huang, J. H. (2014). Impact of microorganisms on arsenic biogeochemistry: A Review. Water, Air and Soil Pollution, 225, 1848.
Huang, Y., Li, H., Rensing, C., Zhao, K., Johnstone, L., & Wang, G. (2012). Genome sequence of the facultative anaerobic arsenite-oxidizing and nitrate-reducing bacterium Acidovorax sp. strain NO1. Journal of Bacteriology, 194, 1635–1636.
Ishii, S., Yamamoto, M., Kikuchi, M., Oshima, K., Hattori, M., Otsuka, S., & Senoo, K. (2009). Microbial populations responsive to denitrification-inducing conditions in rice paddy soil, as revealed by comparative 16S rRNA gene analysis. Applied and Environmental Microbiology, 75, 7070–7078.
Ishii, S., Ashida, N., Otsuka, S., & Senoo, K. (2010). Isolation of oligotrophic denitrifiers carrying previously uncharacterized functional gene sequences. Applied and Environmental Microbiology, 77, 338–345.
Janssen, P. J., Houdt, R. V., Moors, H., Monsieurs, P., Morin, N., Michaux, A., Benotmane, M. A., Leys, N., Vallaeys, T., & Lapidus, A. (2010). The complete genome sequence of Cupriavidus metallidurans strain CH34, a master survivalist in harsh and anthropogenic environments. PLoS One, 5, e10433.
Jin, H., Wang, H., Zhang, Y., Hu, T., Lin, Z., Liu, B., Ma, J., Wang, X., Liu, Q., Lin, X., & Xie, Z. (2020). Description of Azotobacter chroococcum subsp. isscasi subsp. nov. isolated from paddy soil and establishment of Azotobacter chroococcum subsp. chroococcum subsp. nov. International Journal of Systematic and Evolutionary Microbiology, 70, 2124–2131.
Kaiser, E. A., Kohrs, K., Kucke, M., Schnug, E., Heinemeyer, O., & Munch, J. C. (1998). Nitrous oxide release from arable soil: Important of N-Fertilization, crops and temporal variation. Soil Biology & Biochemistry, 30, 1553–1563.
Leloup, J., Quillet, L., Berthe, T., & Petit, F. (2006). Diversity of the dsrAB (dissimilatory sulfite reductase) gene sequences retrieved from two contrasting mudflats of the Seine estuary, France. FEMS Microbiology Ecology, 55, 230–238.
Li, G., Sun, G. X., Williams, P. N., Nunes, L., & Zhu, Y. G. (2011). Inorganic arsenic in Chinese food and its cancer risk. Environment International, 37, 1219–1225.
Li, X., Zhang, L., & Wang, G. (2014). Genomic evidence reveals the extreme diversity and wide distribution of the arsenic-related genes in Burkholderiales. PLoS One, 9, e92236.
Li, X., Zhang, W., Liu, T., Chen, L., Chen, P., & Li, F. (2016). Changes in the composition and diversity of microbial communities during anaerobic nitrate reduction and Fe(II) oxidation at circumneutral pH in paddy soil. Soil Biology & Biochemistry, 94, 70–79.
Li, X., Qiao, J., Li, S., Haggblom, M. M., Li, F., & Hu, M. (2020). Bacterial Communities and functional genes stimulated during anaerobic arsenite oxidation and nitrate reduction in a paddy soil. Environmental Science and Technology, 54, 2172–2181.
Liu, T., Li, X., Zhang, W., Hu, M., & Li, F. (2014a). Fe(III) oxides accelerate microbial nitrate reduction and electricity generation by Klebsiella pneumoniae L17. Journal of Colloid & Interface Science, 423, 25–32.
Liu, T., Zhang, W., Li, X., Li, F., Zhang, W., & Shen, W. (2014b). Kinetics of competitive reduction of nitrate and iron oxides by HS01. Soil Science Society of America Journal, 78, 1903–1912.
Ma, Q., Qu, Y., Zhang, Z., Li, P., & Tang, H. (2015). Genome sequence of an efficient indole-degrading bacterium, Cupriavidus sp. Strain IDO, with potential polyhydroxyalkanoate production applications. Genome Announcements, 3(2), e00102–e00115.
Meharg, A. A. (2004). Arsenic in rice – understanding a new disaster for South-East Asia. Trends in Plant Science, 9, 415–417.
Meharg, A. A., & Zhao, F. J. (2012). Arsenic & rice. Springer. https://doi.org/10.1007/978-94-007-2947-6_1
Mori, K., Suzuki, K., Urabe, T., Sugihara, M., Tanaka, K., Hamada, M., & Hanada, S. (2011). Thioprofundum hispidum sp. nov., an obligately chemolithoautotrophic sulfur-oxidizing gammaproteobacterium isolated from the hydrothermal field on Suiyo Seamount, and proposal of Thioalkalispiraceae fam. nov. in the order Chromatiales. International Journal of Systematic and Evolutionary Microbiology, 61, 2412–2418.
Mukhopadhyay, R., Rosen, B. P., Le, T. P., & Silver, S. (2002). Microbial arsenic: From geocycles to genes and enzymes. FEMS Microbiology Reviews, 26, 311–325.
Nam, J. H., Ventura, J., Yeom, I. T., Lee, Y., & Jahng, D. (2016). A novel perchlorate- and nitrate-reducing bacterium, Azospira sp. PMJ. Applied Microbiology and Biotechnology, 100, 1–14.
Ogawa, K., Akagawa, E., Yamane, K., Sun, Z. W., Lacelle, M., Zuber, P., & Nakano, M. M. (1995). The nasB operon and nasA gene are required for nitrate/nitrite assimilation in Bacillus subtilis. Journal of Bacteriology, 177, 1409–1413.
Oremland, R. S., & Stolz, J. F. (2003). The Ecology of Arsenic. Science, 300, 939–944.
Oremland, R. S., Hoeft, S. E., Santini, J. M., Bano, N., Hollibaugh, R. A., & Hollibaugh, J. T. (2002). Anaerobic oxidation of arsenite in Mono Lake water and by a facultative, arsenite-oxidizing chemoautotroph, strain MLHE-1. Applied and Environmental Microbiology, 68, 4795–4802.
Ouan, Z. X., Im, W. T., & Lee, S. T. (2006). Azonexus caeni sp nov., a denitrifying bacterium isolated from sludge of a wastewater treatment plant. International Journal of Systematic and Evolutionary Microbiology, 56, 1043–1046.
Pérez-Pantoja, D., Iglesia, R. D. L., Pieper, D. H., & González, B. (2008). Metabolic reconstruction of aromatic compounds degradation from the genome of the amazing pollutant-degrading bacterium Cupriavidus necator JMP134. FEMS Microbiology Reviews, 32, 736–794.
Puranik, S., Talkal, R., Qureshi, A., Khardenavis, A., Kapley, A., & Purohit, H. J. (2013). Genome sequence of the pigment-producing bacterium Pseudogulbenkiania ferrooxidans, isolated from Loktak Lake. Genome Announcements, 1, e01115–e1113.
Qiao, J. T., Liu, J. Y., Palomo, A., Bostick, B. C., Phan, K., Zheng, Y., & Li, F. B. (2023). Prevalence of methylated arsenic and microbial arsenic methylation genes in paddy soils of the Mekong Delta. Environmental Science & Technology, 57, 9675–9682.
Rhine, E. D., Phelps, C. D., & Young, L. Y. (2006). Anaerobic arsenite oxidation by novel denitrifying isolates. Environmental Microbiology, 8, 899–908.
Rütting, T., Boeckx, P., Müller, C., & Klemedtsson, L. (2011). Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle. Biogeosciences Discussions, 8, 1779–1791.
Sabra, W., Zeng, A. P., Lunsdorf, H., & Deckwer, W. D. (2000). Effect of oxygen on formation and structure of Azotobacter vinelandii alginate and its role in protecting nitrogenase. Applied and Environmental Microbiology, 66, 4037–4044.
Sakai, S., Imachi, H., Hanada, S., Ohashi, A., Harada, H., & Kamagata, Y. (2008). Methanocella paludicola gen. nov., sp. nov., a methane-producing archaeon, the first isolate of the lineage 'Rice Cluster I’, and proposal of the new archaeal order Methanocellales ord. nov. International Journal of Systematic and Evolutionary Microbiology, 58, 929–936.
Sato, T., & Kobayashi, Y. (1998). The ars operon in the skin element of Bacillus subtilis confers resistance to arsenate and arsenite. Journal of Bacteriology, 180, 1655–1661.
Scheid, D., Stubner, S., & Conrad, R. (2004). Identification of rice root associated nitrate, sulfate and ferric iron reducing bacteria during root decomposition. FEMS Microbiology Ecology, 50, 101–110.
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., Lesniewski, R. A., Oakley, B. B., Parks, D. H., & Robinson, C. J. (2009). Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied & Environmental Microbiology, 75, 7537.
Senn, D. B., & Hemond, H. F. (2002). Nitrate controls on iron and arsenic in an urban lake. Science, 296, 2373–2376.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17, 517–568.
Smith, A. H., Lopipero, P. A., Bates, M. N., & Steinmaus, C. M. (2002). Public health. Arsenic epidemiology and drinking water standards. Science, 296, 2145–2146.
Sonne-Hansen, J., & Ahring, B. K. (1999). Thermodesulfobacterium hveragerdense sp. nov., and Thermodesulfovibrio islandicus sp. nov., two thermophilic sulfate reducing bacteria isolated from a Icelandic hot spring. Systematic & Applied Microbiology, 22, 559–564.
Sun, W., Sierra, R., & Field, J. A. (2008). Anoxic oxidation of arsenite linked to denitrification in sludges and sediments. Water Research, 42, 4569–4577.
Sun, W., Sierra-Alvarez, R., Fernandez, N., Sanz, J. L., Amils, R., Legatzki, A., Maier, R. M., & Field, J. A. (2009). Molecular characterization and in situ quantification of anoxic arsenite-oxidizing denitrifying enrichment cultures. FEMS Microbiology Ecology, 68, 72–85.
Sun, W., Sierra-Alvarez, R., Milner, L., & Field, J. A. (2010). Anaerobic oxidation of arsenite linked to chlorate reduction. Applied and Environmental Microbiology, 76, 6804–6811.
Sun, Z., Lv, Y., Liu, Y., & Ren, R. (2016). Removal of nitrogen by heterotrophic nitrification-aerobic denitrification of a novel metal resistant bacterium Cupriavidus sp. S1. Bioresource Technology, 220, 142–150.
Terry, L. R., Kulp, T. R., Wiatrowski, H., Miller, L. G., & Oremland, R. S. (2015). Microbiological oxidation of antimony(III) with oxygen or nitrate by bacteria isolated from contaminated mine sediments. Applied and Environmental Microbiology, 81, 8478–8488.
Weber, K. A., Hedrick, D. B., Peacock, A. D., Thrash, J. C., White, D. C., Achenbach, L. A., & Coates, J. D. (2009). Physiological and taxonomic description of the novel autotrophic, metal oxidizing bacterium, Pseudogulbenkiania sp. strain 2002. Applied Microbiology and Biotechnology, 83, 555–565.
Weelink, S. A. B., van Doesburg, W., Saia, F. T., Rijpstra, W. I. C., Röling, W. F. M., Smidt, H., & Stams, A. J. M. (2009). A strictly anaerobic betaproteobacterium Georgfuchsia toluolica gen. nov., sp. nov. degrades aromatic compounds with Fe(III), Mn(IV) or nitrate as an electron acceptor. FEMS Microbiology Ecology, 70, 575–585.
Xu, X. Y., Mcgrath, S. P., Meharg, A. A., & Zhao, F. J. (2008). Growing rice aerobically markedly decreases arsenic accumulation. Environmental Science and Technology, 42, 5574–5579.
Yamaguchi, N., Nakamura, T., Dong, D., Takahashi, Y., Amachi, S., & Makino, T. (2011). Arsenic release from flooded paddy soils is influenced by speciation, Eh, pH, and iron dissolution. Chemosphere, 83, 925–932.
Zecchin, S., Mueller, R. C., Seifert, J., Stingl, U., Anantharaman, K., Bergen, M. V., Cavalca, L., & Pester, M. (2018). Rice paddy Nitrospirae encode and express genes related to sulfate respiration: Proposal of the new genus “Candidatus Sulfobium”. Applied and Environmental Microbiology, 84, e02224–e2317.
Zhang, W., Li, X., Liu, T., & Li, F. (2012). Enhanced nitrate reduction and current generation by Bacillus sp. in the presence of iron oxides. Journal of Soils and Sediments, 12, 354–365.
Zhang, W., Li, X., Liu, T., Li, F., & Shen, W. (2014). Competitive reduction of nitrate and iron oxides by Shewanella putrefaciens 200 under anoxic conditions. Colloids & Surfaces A Physicochemical & Engineering Aspects, 445, 97–104.
Zhang, J., Zhou, W., Liu, B., He, J., Shen, Q., & Zhao, F. J. (2015a). Anaerobic arsenite oxidation by an autotrophic arsenite-oxidizing bacterium from an arsenic-contaminated paddy soil. Environmental Science & Technology, 49, 5956–5964.
Zhang, S. Y., Zhao, F. J., Sun, G. X., Su, J. Q., Yang, X. R., Hu, L., & Zhu, Y. G. (2015b). Diversity and abundance of arsenic biotransformation genes in paddy soils from southern China. Environmental Science & Technology, 49, 4138–4146.
Zhang, J., Zhao, S., Xu, Y., Zhou, W., Huang, K., Tang, Z., & Zhao, F. (2017). Nitrate stimulates anaerobic microbial arsenite oxidation in paddy soils. Environmental Science & Technology, 51, 4377–4386.
Zhang, J., Chai, C. W., Thomasarrigo, L. K., Zhao, S. C., & Zhao, F. J. (2020a). Nitrite accumulation is required for microbial anaerobic iron oxidation, but not for arsenite oxidation, in two heterotrophic denitrifiers. Environmental Science & Technology, 54, 4036–4045.
Zhang, M., Li, Z., Haggblom, M. M., Young, L., He, Z., Li, F., Xu, R., Sun, X., & Sun, W. (2020b). Characterization of nitrate-dependent As(III)-oxidizing communities in arsenic-contaminated soil and investigation of their metabolic potentials by the combination of DNA-stable isotope probing and metagenomics. Environmental Science & Technology, 54, 7366–7377.
Funding
This work is supported by the GDAS Special Project of Science and Technology Development (2020GDASYL-20200103053), the National Natural Science Foundation of China (41907130), the Special Topic on Basic and Applied Basic Research of Guangzhou (SL2022A04J01591), Research and Development Program in Key Areas of Guangdong Province (2020B1111530002), and GDAS' Project of Science and Technology Development (2022GDASZH-2022030604).
Author information
Authors and Affiliations
Contributions
Shuang Li and Yinlin Lu: conceived the presented idea. Shuang Li, Jianjun Chen, and Jian Ma contributed to the writing and prepared the figures and tables.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Chen, J., Ma, J. et al. Functional Microbial Communities Involved in As(III) Oxidation Coupled with Nitrate Reduction in a Paddy Soil. Water Air Soil Pollut 234, 598 (2023). https://doi.org/10.1007/s11270-023-06616-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06616-x