Abstract
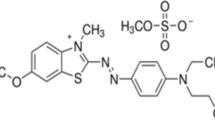
This study involved modifying carbon derived from rubber fruit shells, referred to as nAC-RFs, without activation. The modification process utilized a silane agent known as 3-Aminopropyl-Triethoxysilane (APTES), resulting in the formation of nAC-RFs-S. To confirm the successful modification, the adsorbent underwent characterization using an Infrared Spectrometer (IR) to identify functional groups and Scanning Electron Microscopy-Energy Dispersive-X-ray (SEM–EDX) to analyze surface morphology and constituent elements. The adsorption capacity of nAC-RFs-S was evaluated through batch adsorption experiments involving Coomassie Brilliant Blue (CBB) dye (anionic) and Methylene Blue (MB) dye (cationic). The optimal conditions for CBB and MB adsorption on both nAC-RFs and nAC-RFs-S were found to be pH 5, a contact time of 90 min, and a temperature of 27 °C. The adsorption kinetics of CBB and MB onto nAC-RFs and nAC-RFs-S followed the pseudo-second-order kinetic model. The pseudo-second-order rate constants (k2) for CBB were 2.674 × 10–3 and 0.185 × 10–3 (g/mg·min) for nAC-RFs and nAC-RFs-S, respectively, while for MB, the corresponding values were 2.735 × 10–3 and 0.415 × 10–3 (g/mg·min). CBB adsorption tended to conform to the Freundlich adsorption isotherm model on nAC-RFs, whereas, on nAC-RFs-S, it tended to align with the Langmuir adsorption isotherm model. For MB, both nAC-RFs and nAC-RFs-S exhibited adsorption tendencies that followed the Freundlich isotherm model. The nAC-RFs-S adsorbent demonstrated remarkable effectiveness in adsorbing CBB and MB in solution, with the ability to be reused for up to three cycles while maintaining an adsorption percentage exceeding 80%. Consequently, the nAC-RFs-S adsorbent holds promise as an advantageous and efficient solution for combating water pollution caused by toxic chemicals like synthetic dyes.









Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Abdel-Ghani, N. T., El-Chaghaby, G. A., Rawash, E. S. A., & Lima, E. C. (2017). Adsorption of coomassie brilliant blue r-250 dye onto novel activated carbon prepared from nigella sativa l. waste: equilibrium, kinetics and thermodynamics running title: adsorption of brilliant blue dye onto Nigella sativa L. waste activated carbon. Journal of the Chilean Chemical Society, 62(2), 3505–3511. https://doi.org/10.4067/S0717-97072017000200016
Ahmad, A., Razali, M. H., Mamat, M., Mehamod, F. S. B., & Amin, K. A. M. (2017). Adsorption of methyl orange by synthesized and functionalized-CNTs with 3-Aminopropyltriethoxysilane loaded TiO2 nanocomposites. Chemosphere, 168, 474–482. https://doi.org/10.1016/j.chemosphere.2016.11.028
Ai, L., Zhang, C., Liao, F., Wang, Y., Li, M., Meng, L., & Jiang, J. (2011). Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. Journal of Hazardous Materials, 198, 282–290. https://doi.org/10.1016/j.jhazmat.2011.10.041
Al-Hazmi, G. H., Akhdhar, A., Shahat, A., & Elwakeel, K. Z. (2022). Adsorption of gentian violet dye onto mesoporous aluminosilica monoliths: Nanoarchitectonics and application to industrial wastewater. International Journal of Environmental Analytical Chemistry. https://doi.org/10.1080/03067319.2022.2104641
Álvarez-Torrellas, S., Muñoz, M., Zazo, J. A., Casas, J. A., & García, J. (2016). Synthesis of high surface area carbon adsorbents prepared from pine sawdust-Onopordum acanthium L. for nonsteroidal antiinflammatory drugs adsorption. Journal of Environmental Management, 183, 294–305. https://doi.org/10.1016/j.jenvman.2016.08.077
An, L., Si, C., Bae, J. H., Jeong, H., & Kim, Y. S. (2020). One-step silanization and amination of lignin and its adsorption of Congo red and Cu(II) ions in aqueous solution. International Journal of Biological Macromolecules, 159(2020), 222–230. https://doi.org/10.1016/j.ijbiomac.2020.05.072
Atar, N., Olgun, A., & Wang, S. (2012). Adsorption of cadmium (II) and zinc (II) on boron enrichment process waste in aqueous solutions: Batch and fixed-bed system studies. Chemical Engineering Journal, 192(3), 1–7. https://doi.org/10.1016/j.cej.2012.03.067
Bhavyasree, P. G., & Xavier, T. S. (2021). Adsorption studies of methylene blue, coomassie brilliant blue, and congo red dyes onto CuO/C nanocomposites synthesized via vitex negundo linn leaf extract. Current Research in Green and Sustainable Chemistry, 4, 100161. https://doi.org/10.1016/j.crgsc.2021.100161
Buhani, Rinawati, Suharso, Yuliasari, D. P., & Yuwono, S. D. (2017). Removal of Ni(II), Cu(II), and Zn(II) ions from aqueous solution using Tetraselmis sp. biomass modified with silica-coated magnetite nanoparticles. Desalination and Water Treatment, 80, 203–213. https://doi.org/10.5004/dwt.2017.20932
Buhani, Hariyanti, F., Suharso, Rinawati, & Sumadi. (2019). Magnetized algae-silica hybrid from porphyridium sp. biomass with Fe3O4 particle and its application as adsorbent for the removal of methylene blue from aqueous solution. Desalination and Water Treatment, 142, 331–340. https://doi.org/10.5004/dwt.2019.23533
Buhani, S., Rilyanti, M., Sari, M., & Sumadi. (2021a). Removal of Cd(II) ions in solution by activated carbon from palm oil shells modified with magnetite. Desalination and Water Treatment, 218, 352–362. https://doi.org/10.5004/dwt.2021.26978
Buhani, Wijayanti, T. A., Suharso, Sumadi, & Ansori, M. (2021b). Application of modified green algae Nannochloropsis sp. as adsorbent in the simultaneous adsorption of methylene blue and Cu(II) cations in solution. Sustainable Environment Research, 31(17), 1–12. https://doi.org/10.1186/s42834-021-00090-y
Buhani, S., Rilyanti, M., Devi, F., Antika, R., Puji, L., & Sumadi, L. (2023). Functionalization of carbon from rubber fruit shells (Hevea brasiliensis) with silane agents and its application to the adsorption of bi - component mixtures of methylene blue and crystal violet. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-023-28031-9
Can, K., Ozmen, M., & Ersoz, M. (2009). Immobilization of albumin on aminosilane modified superparamagnetic magnetite nanoparticles and its characterization. Colloids and Surfaces B: Biointerfaces, 71(1), 154–159. https://doi.org/10.1016/j.colsurfb.2009.01.021
Çelekli, A., & Bozkurt, H. (2013). Predictive modeling of an azo metal complex dye sorption by pumpkin husk. Environmental Science and Pollution Research, 20(10), 7355–7366. https://doi.org/10.1007/s11356-013-1751-5
Elgarahy, A. M., Elwakeel, K. Z., Elshoubaky, G. A., & Mohammad, S. H. (2019). Untapped sepia shell-based composite for the sorption of cationic and anionic dyes. Water, Air, & Soil Pollution, 230, 217. https://doi.org/10.1007/s11270-019-4247-1
Elgarahy, A. M., Elwakeel, K. Z., Mohammad, S. H., & Elshoubaky, G. A. (2021). A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Cleaner Engineering and Technology, 4, 100209. https://doi.org/10.1016/j.clet.2021.100209
Elwakeel, K. Z., Shahat, A., Khan, Z. A., Alshitari, W., & Guibal, E. (2020). Magnetic metal oxide-organic framework material for ultrasonic-assisted sorption of titan yellow and rose bengal from aqueous solutions. Chemical Engineering Journal, 392, 123635. https://doi.org/10.1016/j.cej.2019.123635
Fajriyah, N. S., Buhani, B., & Suharso, S. (2023). Adsorption kinetics and isotherm of crystal violet by carbon modified with magnetite (Fe3O4) and triethoxyphenylsilane (TEPS) from rubber fruit shell. Indonesian Journal of Chemistry, 23(1), 170–183. https://doi.org/10.22146/ijc.76201
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochem, 34(5), 451–465. https://doi.org/10.1016/S0032-592(98)00112-5
Huang, C., & Hu, B. (2008). Silica-coated magnetic nanoparticles modified with γ-Mercaptopropyltrimethoxysilane for fast and selective solid phase extraction of trace amounts of Cd, Cu, Hg, and Pb in environmental and biological samples prior to their determination by inductively Co. Spectrochimica Acta - Part B Atomic Spectroscopy, 63(3), 437–444. https://doi.org/10.1016/j.sab.2007.12.010
Ibrahim, H. K., Al-Da’ Amy, M. A., & Kreem, E. T. (2019). Decolorization of coomassie brilliant blue G-250 dye using snail shell powder by action of adsorption processes. Research Journal of Pharmacy and Technology, 12(10), 4921–4925. https://doi.org/10.5958/0974-360X.2019.00853.9
Iwuozor, K. O., Ighalo, J. O., Ogunfowora, L. A., Adeniyi, A. G., & Igwegbe, C. A. (2021). An empirical literature analysis of adsorbent performance for methylene blue uptake from aqueous media. Journal of Environmental Chemical Engineering, 9(4), 105658. https://doi.org/10.1016/j.jece.2021.105658
Jamwal, H. S., Kumari, S., Chauhan, G. S., Reddy, N. S., & Ahn, J. H. (2017). Silica-polymer hybrid materials as methylene blue adsorbents. Journal of Environmental Chemical Engineering, 5(1), 103–113. https://doi.org/10.1016/j.jece.2016.11.029
Kanakaraju, D., Ravichandar, S., & Lim, Y. C. (2017). Combined effects of adsorption and photocatalysis by hybrid TiO2/ZnO-calcium alginate beads for the removal of copper. Journal of Environmental Sciences (China), 55, 214–223. https://doi.org/10.1016/j.jes.2016.05.043
Katheresan, V., Kansedo, J., & Lau, S. Y. (2018). Efficiency of various recent wastewater dye removal methods: A review. Journal of Environmental Chemical Engineering, 6(4), 4676–4697. https://doi.org/10.1016/j.jece.2018.06.060
Kosa, S. A., Al-Zhrani, G., & Abdel Salam, M. (2012). Removal of heavy metals from aqueous solutions by multi-walled carbon nanotubes modified with 8-Hydroxyquinoline. Chemical Engineering Journal, 181–182, 159–168. https://doi.org/10.1016/j.cej.2011.11.044
Lee, S., Jeong, J., Shin, S., Kim, J. C., & Kim, J. D. (2004). Synthesis and characterization of superparamagnetic maghemite nanoparticles prepared by coprecipitazion technique. Journal of Magnetism and Magnetic Materials, 282, 147–150. https://doi.org/10.1016/j.jmmm.2004.04.035
Liang, X., Lu, Y., Li, Z., Yang, C., Niu, C., & Su, X. (2017). Bentonite/carbon composite as highly recyclable adsorbents for alkaline wastewater treatment and organic dye removal. Microporous and Mesoporous Materials, 241, 107–114. https://doi.org/10.1016/j.micromeso.2016.12.016
Liu, Y., Li, Y., Li, X. M., & He, T. (2013). Kinetics of (3-Aminopropyl)triethoxylsilane (APTES) silanization of superparamagnetic iron oxide nanoparticles. Langmuir, 29(49), 15275–15282. https://doi.org/10.1021/la403269u
Moosavi, S., Lai, C. W., Gan, S., Zamiri, G., Akbarzadeh Pivehzhani, O., & Johan, M. R. (2020). Application of efficient magnetic particles and activated carbon for dye removal from wastewater. ACS Omega, 5(33), 20684–20697. https://doi.org/10.1021/acsomega.0c01905
Munguía-Cortés, L., Pérez-Hermosillo, I., Ojeda-López, R., Esparza-Schulz, J. M., Felipe-Mendoza, C., Cervantes-Uribe, A., & Domínguez-Ortiz, A. (2017). APTES-functionalization of SBA-15 using ethanol or toluene: Textural characterization and sorption performance of carbon dioxide. Journal of the Mexican Chemical Society, 61(4), 273–281. https://doi.org/10.29356/jmcs.v61i4.457
Priya, Kaith, B. S., Shanker, U., Gupta, B., & Bhatia, J. K. (2018). RSM-CCD optimized in-air synthesis of photocatalytic nanocomposite: Application in removal-degradation of toxic brilliant blue. Reactive and Functional Polymers, 131(107), 122. https://doi.org/10.1016/j.reactfunctpolym.2018.07.016
Radoičić, M., Šaponjić, Z., Nedeljković, J., Ćirić-Marjanović, G., & Stejskal, J. (2010). Self-assembled polyaniline nanotubes and nanoribbons/titanium dioxide nanocomposites. Synthetic Metals, 160(11–12), 1325–1334. https://doi.org/10.1016/j.synthmet.2010.04.010
Rocha, L. S., Pereira, D., Sousa, É., Otero, M., Esteves, V. I., & Calisto, V. (2020). Recent advances on the development and application of magnetic activated carbon and char for the removal of pharmaceutical compounds from waters: A review. Science of the Total Environment, 718, 137272. https://doi.org/10.1016/j.scitotenv.2020.137272
Sahoo, S. K., Panigrahi, G. K., Sahoo, J. K., Pradhan, A. K., Purohit, A. K., & Dhal, J. P. (2021). Electrospun magnetic polyacrylonitrile-GO hybrid nanofibers for removing Cr(VI) from water. Journal of Molecular Liquids, 326, 115364. https://doi.org/10.1016/j.molliq.2021.115364
Shao, Y., Zhou, L., Bao, C., Ma, J., Liu, M., & Wang, F. (2016). Magnetic responsive metal–organic frameworks nanosphere with core–shell structure for highly efficient removal of methylene blue. Chemical Engineering Journal, 283, 1127–1136. https://doi.org/10.1016/j.cej.2015.08.051
Sharma, G., Naushad, M., Kumar, A., Rana, S., Sharma, S., Bhatnagar, A., Stadler, F. J., Ghfar, A. A., & Khan, M. R. (2017). Efficient removal of coomassie brilliant blue R-250 dye using starch/poly(alginic acid-cl-acrylamide) nanohydrogel. Process Safety and Environmental Protection, 109, 301–310. https://doi.org/10.1016/j.psep.2017.04.011
Thamer, B. M., Aldalbahi, A., Moydeen, A. M., El-Hamshary, H., Al-Enizi, A. M., & El-Newehy, M. H. (2019). Effective adsorption of coomassie brilliant blue dye using poly(phenylene diamine)grafted electrospun carbon nanofibers as a novel adsorbent. Materials Chemistry and Physics, 234(133), 145. https://doi.org/10.1016/j.matchemphys.2019.05.087
Wong, K. T., Yoon, Y., & Jang, M. (2015). Enhanced recyclable magnetized palm shell waste-based powdered activated carbon for the removal of ibuprofen: Insights for kinetics and mechanisms. PLoS One, 10(10), 1–18. https://doi.org/10.1371/journal.pone.0141013
Wong, K. T., Yoon, Y., Snyder, S. A., & Jang, M. (2016). Phenyl-functionalized magnetic palm-based powdered activated carbon for the effective removal of selected pharmaceutical and endocrine-disruptive compounds. Chemosphere, 152, 71–80. https://doi.org/10.1016/j.chemosphere.2016.02.090
Yilmaz, M. S. (2022). Graphene oxide/hollow mesoporous silica composite for selective adsorption of methylene blue. Microporous Mesoporous Mater, 330, 111570. https://doi.org/10.1016/j.micromeso.2021.111570
Zulaicha, A. S., Saputra, I. S., Buhani, B., & Suharso, S. (2022). Magnetite particle coating to activated carbon of oil palm shells as adsorbent of Cu(II) and Ni(II) cation. Journal of the Iranian Chemical Society, 19(12), 4777–4787. https://doi.org/10.1007/s13738-022-02641-5
Acknowledgements
The authors express their gratitude to the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia (KemendikbudRistek) for providing funding for this project under contract number: 027/E5/PG.02.00.PL/2023. We also acknowledge the support of the Research and Community Service Institute of the University of Lampung (LPPM Universitas Lampung) for their assistance in conducting this research.
Funding
This work was funded by Ministry of Education, Culture, Research and Technology (Kemdikbudristek) of the Republic of Indonesia with contract number: 027/E5/PG.02.00.PL/2023.
Author information
Authors and Affiliations
Contributions
Buhani: Conceptualization, methodology, Investigation, Data curation, and writing original draft.
Jilda Sofiana Dewi: Methodology, Investigation, and Data curation.
Nadya Syarifatul Fajriyah: Methodology, Investigation, and Data curation.
Mita Rilyanti: Methodology, Investigation, Data curation, and Data analysis.
Suharso: Conceptualization, methodology, Investigation, and Data curation.
Sumadi: Conceptualization, Data curation, Data analysis, and editing.
Khalid Z. Elwakeel: Review and editing.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Buhani, Dewi, J.S., Fajriyah, N.S. et al. Modification of Non-Activated Carbon from Rubber Fruit Shells with 3-(Aminopropyl)-Triethoxysilane and Its Adsorption Study on Coomassie Brilliant Blue and Methylene Blue in Solution. Water Air Soil Pollut 234, 578 (2023). https://doi.org/10.1007/s11270-023-06506-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06506-2