Abstract
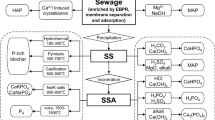
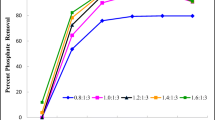
Phosphorus can only be obtained from phosphate ore, and the price of phosphate ore is gradually increasing because of the limited phosphate ore reserves. The global population is continually increasing and is predicted to rise to approximately 9.1 billion people by 2040. Therefore, phosphorus is being increasingly consumed because it is an essential component of fertilizer. Numerous studies have researched methods to recover phosphorus. Among these methods, the crystallization technique has the most advantages compared to other methods (chemical, physical, and biological treatments). Struvite and calcium phosphate crystals are typically used for phosphorus recovery. Struvite was created by combining a 1:1:1 molar ratio of NH4+, PO43−, and Mg2+. For struvite crystallization, we performed diverse experiments focusing on pH range, seed types, and the injection amount of various chemicals (CaO and MgO). We found that a pH of 9 and injecting 0.3 g of CaO and MgO into 240 mL of wastewater were ideal for struvite crystallization. We also performed experiments on calcium phosphate crystallization using calcium silicate hydrate (CSH) compared to other calcium compounds and found that CSH was the best compound to use for phosphate crystallization.
















Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Acelas, N. Y., Flórez, E., & López, D. (2015). Phosphorus recovery through struvite precipitation from wastewater: Effect of the competitive ions. Desalination and Water Treatment, 54(9), 2468–2479. https://doi.org/10.1080/19443994.2014.902337
Amar, H., Benzaazoua, M., Elghali, A., Hakkou, R., & Taha, Y. (2022). Waste rock reprocessing to enhance the sustainability of phosphate reserves: A critical review. Journal of Cleaner Production, 381(P1), 135151. https://doi.org/10.1016/j.jclepro.2022.135151
Bahsaine, K., Mekhzoum, M. E. M., Benzeid, H., el kacem Qaiss, A., & Bouhfid, R. (2022). Recent progress in heavy metals extraction from phosphoric acid: A short review. Journal of Industrial and Engineering Chemistry, 115(120), 134. https://doi.org/10.1016/j.jiec.2022.08.029
Beheshti, M., Alikhani, H. A., Pourbabaee, A. A., Etesami, H., Asadi Rahmani, H., & Noroozi, M. (2022). Enriching periphyton with phosphate-solubilizing microorganisms improves the growth and concentration of phosphorus and micronutrients of rice plant in calcareous paddy soil. Rhizosphere, 24(July), 100590. https://doi.org/10.1016/j.rhisph.2022.100590
Filippelli, G. M. (2011). Phosphate rock formation and marine phosphorus geochemistry: The deep time perspective. Chemosphere, 84(6), 759–766. https://doi.org/10.1016/j.chemosphere.2011.02.019
Gebremariam, S. Y., Beutel, M. W., Christian, D., & Hess, T. F. (2011). Research advances and challenges in the microbiology of enhanced biological phosphorus removal-A critical review. Water Environment Research, 83(3), 195–219. https://doi.org/10.2175/106143010x12780288628534
Gondek, M., Weindorf, D. C., Thiel, C., & Kleinheinz, G. (2020). Soluble salts in compost and their effects on soil and plants: A review. Taylor and Francis Inc. https://doi.org/10.1080/1065657X.2020.1772906
Guan, W., Ji, F. Y., Chen, Q. K., Yan, P., & Zhou, W. W. (2014a). Phosphorus recovery using porous calcium silicate hydrate as seed crystal in form of hydroxyapatite. Materials Research Innovations, 18(1), 43–49. https://doi.org/10.1179/1433075X13Y.0000000112
Guan, Wei, Ji, F., Fang, D., Cheng, Y., Fang, Z., Chen, Q., & Yan, P. (2014). Porosity formation and enhanced solubility of calcium silicate hydrate in hydrothermal synthesis. Ceramics International, 40(1 PART B), 1667–1674. https://doi.org/10.1016/j.ceramint.2013.07.058
Heckenmüller, M., Narita, D., & Klepper, G. (2014). Global availability of phosphorus and its implications for global food supply: An economic overview. Kiel Working Papers, (1897), 26. https://www.econstor.eu/bitstream/10419/90630/1/776834355.pdf
Heffer, P., & Prud’homme, M. (2016). Global nitrogen fertilizer demand and supply: Trend, current level and outlook. International Fertilizer Association (IFA). 7th International Nitrogen Initiative Conference, (4–8, December), 1–11. https://www.ini2016.com/
Jellali, S., Wahab, M. A., Hassine, R. B., Hamzaoui, A. H., & Bousselmi, L. (2011). Adsorption characteristics of phosphorus from aqueous solutions onto phosphate mine wastes. Chemical Engineering Journal, 169(1–3), 157–165. https://doi.org/10.1016/j.cej.2011.02.076
Jiang, J. Q., & Wu, L. (2010). Preliminary study of calcium silicate hydrate (tobermorite) as crystal material to recovery phosphate from wastewater. Desalination and Water Treatment, 23(1–3), 49–54. https://doi.org/10.5004/dwt.2010.1866
Kataki, S., West, H., Clarke, M., & Baruah, D. C. (2016). Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resources, Conservation and Recycling, 107, 142–156. https://doi.org/10.1016/j.resconrec.2015.12.009
Kołodziej, B., Sugier, D., & Kwiatkowski, C. (2015). Phosphorus fertilization and inflorescence removal in American ginseng culture. Industrial Crops and Products, 74, 619–627. https://doi.org/10.1016/j.indcrop.2015.05.047
Li, L., Takahashi, N., Kaneko, K., Shimizu, T., & Takarada, T. (2015). A novel method for nickel recovery and phosphorus removal from spent electroless nickel-plating solution. Separation and Purification Technology, 147, 237–244. https://doi.org/10.1016/j.seppur.2015.04.029
Liu, R., Yang, Z., Wang, G., Xian, J., Li, T., Pu, Y., et al. (2021). Simultaneous removal of ammonium and phosphate in aqueous solution using Chinese herbal medicine residues: Mechanism and practical performance. Journal of Cleaner Production, 313(March), 127945. https://doi.org/10.1016/j.jclepro.2021.127945
Loganathan, P., Vigneswaran, S., Kandasamy, J., & Bolan, N. S. (2014). Removal and recovery of phosphate from water using sorption. Critical Reviews in Environmental Science and Technology, 44(8), 847–907. https://doi.org/10.1080/10643389.2012.741311
Mukherjee, D., Ray, R., & Biswas, N. (2020). Mining phosphate from wastewater: Treatment and reuse. Sustaining Resources for Tomorrow. https://doi.org/10.1162/leon_r_02018
O’Driscoll, C., Rodgers, M., O’Connor, M., Asam, Z. U. Z., De Eyto, E., Poole, R., & Xiao, L. (2011). A potential solution to mitigate phosphorus release following clearfelling in peatland forest catchments. Water, Air, and Soil Pollution, 221(1–4), 1–11. https://doi.org/10.1007/s11270-011-0764-2
Okano, K., Uemoto, M., Kagami, J., Miura, K., Aketo, T., Toda, M., et al. (2013). Novel technique for phosphorus recovery from aqueous solutions using amorphous calcium silicate hydrates (A-CSHs). Water Research, 47(7), 2251–2259. https://doi.org/10.1016/j.watres.2013.01.052
Papargyropoulou, E., Lozano, R., Steinberger, J. K., Wright, N., & bin Ujang, Z. (2014). The food waste hierarchy as a framework for the management of food surplus and food waste. Journal of Cleaner Production, 76(106), 115. https://doi.org/10.1016/j.jclepro.2014.04.020
Qiao, Y., Yin, L., Wang, B., Ke, Q., Deng, X., & Wang, S. (2019). Melatonin promotes plant growth by increasing nitrogen uptake and assimilation under nitrogen deficient condition in winter wheat. Plant Physiology and Biochemistry, 139(26), 342–349. https://doi.org/10.1016/j.plaphy.2019.03.037
Reijnders, L. (2014). Phosphorus resources, their depletion and conservation, a review. Resources, Conservation and Recycling, 93, 32–49. https://doi.org/10.1016/j.resconrec.2014.09.006
Rusan, M. J. M., Albalasmeh, A. A., & Malkawi, H. I. (2016). Treated olive mill wastewater effects on soil properties and plant growth. Water, Air, and Soil Pollution, 227(5), 1–10. https://doi.org/10.1007/s11270-016-2837-8
Tang, S., & Fei, X. (2021). Refractory calcium phosphate-derived phosphorus fertilizer based on hydroxyapatite nanoparticles for nutrient delivery. ACS Applied Nano Materials, 4(2), 1364–1376. https://doi.org/10.1021/acsanm.0c02921
Tran, A. T. K., Zhang, Y., De Corte, D., Hannes, J. B., Ye, W., Mondal, P., et al. (2014). P-recovery as calcium phosphate from wastewater using an integrated selectrodialysis/crystallization process. Journal of Cleaner Production, 77, 140–151. https://doi.org/10.1016/j.jclepro.2014.01.069
Umar, M., Roddick, F., & Fan, L. (2015). Recent advancements in the treatment of municipal wastewater reverse osmosis concentrate - An overview. Critical Reviews in Environmental Science and Technology, 45(3), 193–248. https://doi.org/10.1080/10643389.2013.852378
Wang, Q., & Li, Y. (2010). Phosphorus adsorption and desorption behavior on sediments of different origins. Journal of Soils and Sediments, 10(6), 1159–1173. https://doi.org/10.1007/s11368-010-0211-9
Wang, X. X., Wu, Y. H., Zhang, T. Y., Xu, X. Q., Dao, G. H., & Hu, H. Y. (2016). Simultaneous nitrogen, phosphorous, and hardness removal from reverse osmosis concentrate by microalgae cultivation. Water Research, 94, 215–224. https://doi.org/10.1016/j.watres.2016.02.062
Wu, K. C., Yau, Y. H., & Sze, E. T. P. (2020). Application of anaerobic bacterial ammonification pretreatment to microalgal food waste leachate cultivation and biofuel production. Marine Pollution Bulletin, 153(September 2019), 111007. https://doi.org/10.1016/j.marpolbul.2020.111007
Acknowledgements
This study was supported by Research Program to Solve Urgent Safety Issues of the National Research Foundation of Korea (NRF) funded by the Korean government (Ministry of Science and ICT (MSIT)) (2021M3E9A1103514) and an institutional program grant (2E32442) from the Korea Institute of Science and Technology. This work was also supported by the Korea Environment Industry & Technology Institute (KEITI) through the Subsurface Environmental Pollution Risk Management Technology Development Project funded by the Korea Ministry of Environment (MOE) (ARQ202101728001).
Author information
Authors and Affiliations
Contributions
H.-G. Kim: Writing—original draft, methodology, and investigation.
B. Yang: Formal analysis and methodology
K.-W. Jung: Methodology and validation
S. Lee: Writing—review and validation
J.-W. Choi: Writing—original draft, review and editing, conceptualization, and funding acquisition
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, HG., Yang, B., Jung, KW. et al. Reuse Rate Improvement of Wastewater Through Effective Removal and Recovery of High Concentration Phosphorus in Reverse Osmosis Concentrate and Food Waste Leachates. Water Air Soil Pollut 234, 478 (2023). https://doi.org/10.1007/s11270-023-06486-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06486-3