Abstract
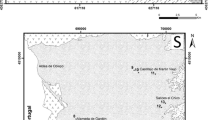
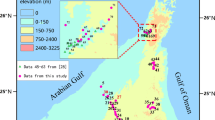
This research was conducted in Amman-Zarqa basin (Upper Cretaceous system) which hosts the main aquifer of Jordan. The region has intensive anthropogenic activities and the geology is dominated by phosphate rocks, which contain high concentrations of uranium. The study aimed to evaluate the water quality and the radioactivity exposure from intake of radionuclides contained in water and assessed through total alpha, total beta, 238U, and 232Th activity concentrations. Groundwater samples were collected from 29 boreholes for determination of major ions and radionuclide concentrations. The sampling was carried out during the rainy season (February) and dry season (June) of 2019 in order to assess the seasonal variation of analyte concentrations. The mean concentrations of major ions varied between seasons as follows: chloride (Cl−1) 162 to 180 µg L−1, sulfate (SO4−2) 47 to 57 µg L−1, bicarbonate (HCO3−1) 288 to 313 µg L−1, and nitrate (NO3−) 42 to 50 µg L−1, in February and June respectively. The ion concentrations of potassium (K+), sodium (Na+), magnesium (Mg+2), nitrate (NO3−), sulphate (SO4−2), and chloride (Cl−) as well as radioactivity levels were within regulatory limits, whereas calcium (Ca+2) and bicarbonate (HCO3−1) concentrations were higher than the recommended limits in national legislation. Statistical test (ANOVA) results indicated significant differences between seasons for Mg+2, NO3−, SO4−2 concentrations. The ion chemistry of groundwater was dominated by HCO3−, Cl−, Na+ and Ca+2, with minor contributions from SO4−2, NO3−, K+ and Mg+2. The annual effective dose due to ingestion of groundwater radionuclides, based on total alpha and total beta activity averaged 0.49 mSv y−1 corresponding to a risk of an extra cancer over lifetime of 25 × 10–4, which is comparable to the national admissible cancer risk limit of 25 × 10–4. The results of this study were compared with national and international water quality standards. Several water parameters attained the recommended limits for the protection of public health, and a thorough water quality monitoring programme and enhanced measures for protection of the aquifer are recommended.



















Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdurabu, W. A., Saleh, M. A., Ramli, A. T., & Heryansyah, A. (2016). Occurrence of natural radioactivity and corresponding health risk in groundwater with an elevated radiation background in Juban District. Yemen Environmental Earth Sciences, 75, 1360.
Abu-Jaber, N., & El-Naser, H. (2016). Geology and hydrochemistry of the deep sandstone aquifers of Jordan. Environmental Earth Sciences, 75, 875.
Al Kuisi, M., & Abdel-Fattah, A. (2010). Groundwater vulnerability to selenium in semi-arid environments: Amman Zarqa Basin. Jordan Environ Geochem Health, 32, 107–128.
Al Qatarneh, G. N., Al Smadi, B., Al-Zboon, K., & Shatanawi, K. M. (2018). Impact of Climate Change on Water Resources in Jordan: A Case Study of Azraq Basin Applied Water Science, 8, 1–14.
AL-Ariqi, W. S., & Ghaleb, A. A. (2010). Assessment of hydrochemical quality of ground water under some urban areas within sana’a secreteriat. Eclética Química, 35, 77–84.
Al-Qaisi BM (2010) Climate change effects on water resources in Amman Zarqa Basin—Jordan Individual Project Report for Climate Change Mitigation and Adaptation, MWI, Amman
Alley, W. M. (1993). Regional ground-water quality. John Wiley & Sons.
Alnawafleh, H., Tarawneh, K., & Alrawashdeh, R. (2013). Geologic and economic potentials of minerals and industrial rocks in Jordan. Natural Science, 5, 756.
Alomari, A. H., Saleh, M. A., Hashim, S., Al-Hada, N., Abukashabeh, A., Alsayaheen, A., & Hamad, M. (2020). Radiological dose and health impact to Jordanian populace due to radioactivity in staple food crops from four representative soils in Jordan. Journal of Radioanalytical and Nuclear Chemistry, 326, 1679–1689. https://doi.org/10.1007/s10967-020-07461-6
Alomari AH, Saleh MA, Hashim S, Alsayaheen A, Abdeldin I (2019a) 238U and 232Th isotopes in groundwater of Jordan: Geological influence, water chemistry, and health impact Radiation Physics and Chemistry:108660
Alomari AH, Saleh MA, Hashim S, Alsayaheen A, Abdeldin I (2019b) Activity concentrations of 226 Ra, 228 Ra, 222 Rn and their health impact in the groundwater of Jordan Journal of Radioanalytical and Nuclear Chemistry:1–14
Aneesha K, Sethulekshmi C, Latha C (2019) Seasonal variation in physico-chemical and microbiological parameters in ground water quality of Anthikkad, Thrissur, Kerala
APHA (2012) standard methods for the examination of water and wastewater. book. American Public Health Association, American Water Works Association, Water environment federation, United states of America
Awad, M. (1997). Environmental Study of the Amman-Zerqa Basin Jordan Environ Geol, 33, 54–60.
Bajjali W, Al-Hadidi K Hydrochemical evaluation of groundwater in Azraq Basin, Jordan using environmental isotopes and GIS techniques. In: 25th annual ESRI international user conference, San Diego, California, 2005. pp 25–29
Bender F (1974) Geology of Jordan vol 7. Natural resources authority and German geological mission in Jordan,, Berlin, Germany
Brugge D, Buchner V (2011) Health effects of uranium: new research findings
Carvalho FP, Madruga MJ, Oliveira JM, Lopes I, Ferrador G, Sequeira M Naturally‐Occurring Radionuclides In Drinking Water From Surface And Groundwater Reservoirs. In: AIP Conference Proceedings, 2008. vol 1. American Institute of Physics, pp 224–229
Carvalho, F., & Fajgelj, A. (2013). Radioactivity in drinking water: routine monitoring and emergency response. Water, Air, and Soil Pollution, 224, 1–7.
Chapman, D. V. (1996). Water quality assessments: A guide to the use of biota, sediments and water in environmental monitoring. CRC Press.
Cothern, C. R., & Ap, R. (1990). Radon, radium, and uranium in drinking water. Lewis publishers.
Dippong T, Mihali C, Ardelean G (2019) Seasonal variation of physico-chemical parameters in the drinking water supply network of Satu Mare city, NW Romania Environmental Engineering & Management Journal (EEMJ) 18
Dinh Chau, N., Dulinski, M., Jodlowski, P., Nowak, J., Rozanski, K., Sleziak, M., & Wachniew, P. (2011). Natural radioactivity in groundwater–a review Isotopes. Environ Health Stud, 47, 415–437.
EFSA. (2009). Uranium in foodstuffs, in particular mineral water. EFSA Journal, 7, 1018.
Fisher, R. S., & Mullican, W. F., III. (1997). Hydrochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the northern Chihuahuan Desert. Trans-Pecos, Texas, USA Hydrogeology Journal, 5, 4–16.
Gedeon R, Amro H, Jawawdeh J, Kilani S, Smith B (1995) Natural radioisotopes in groundwater from the Amman-Zarka basin Jordan. Hydrochemical and regulatory implications. Applications of tracers in arid zone hydrology. Wallingford, UK International Association of Hydrological Sciences 232
Gilli, G., Corrao, G., & Favilli, S. (1984). Concentrations of nitrates in drinking water and incidence of gastric carcinomas: first descriptive study of the Piemonte Region. Italy Sci Total Environ, 34, 35–48.
Gilmore G (2008) practical Gamma-ray spectrometry. 2nd edition edn. John wiley and sons Ltd, UK
Hammouri, N., Adamowski, J., Freiwan, M., & Prasher, S. (2017). Climate change impacts on surface water resources in arid and semi-arid regions: a case study in northern Jordan Acta Geodaetica et. Geophysica, 52, 141–156.
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water vol 2254. Department of the Interior, US Geological Survey,
Idoko, M., & Oklo, A. (2012). Seasonal variation in physico-chemical characteristics of rural groundwater of Benue State. Nigeria Journal of Asian Scientific Research, 2, 574.
Jagadeesh, K., Somashekar, R., & Chandrashekar, J. (2012). seasonal variation of physicochemical characte-ristics and statistical study of groundwater in the hipparagi irrigation command area, karnataka, india. Electronic Journal of Environmental Sciences., 5, 13–20.
Jameel, A., & Hussain, A. (2007). Assessment of ground water quality on banks of Uyyakondan channel of river Cauvery at Tiruchirappalli. Indian Journal of Environmental Protection, 27, 713.
JISM (2008) Jordanian drinking water standard 5th edn. Jordan Institute of Standards and Metrology, Hashmite Kingdom of Jordan
Kim, Y.-s, Park, H.-s, Kim, J.-y, Park, S.-k, Cho, B.-w, Sung, I.-h, & Shin, D.-c. (2004). Health risk assessment for uranium in Korean groundwater. Journal of Environmental Radioactivity, 77, 77–85.
Kumar, P. S. (2013). Interpretation of groundwater chemistry using piper and Chadha’s diagrams: a comparative study from Perambalur Taluk Elixir International Journal. Geoscience, 54, 12208–12211.
Kumar, P. S., Jose, A., & James, E. (2013). Spatial and seasonal variation in groundwater quality in parts of Cuddalore District. South India National Academy Science Letters, 36, 167–179.
Kumar, P. S., Kokkat, A., Kurian, P., & James, E. (2020). Nutrient chemistry and seasonal variation in the groundwater quality of a Riverine Island on the west coast of Kerala. India Sustainable Water Resources Management, 6, 1–8.
Langmuir, D. (1978). Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochimica Et Cosmochimica Acta, 42, 547–569.
Mahlknecht, J., Steinich, B., & De León, I. N. (2004). Groundwater chemistry and mass transfers in the Independence aquifer, central Mexico, by using multivariate statistics and mass-balance models. Environ Geol, 45, 781–795.
Matini, L., Tathy, C., & Moutou, J. (2012). Seasonal groundwater quality variation in Brazzaville. Congo Research Journal of Chemical Sciences, 2231, 606X.
Moh’d, B. K., & Powell, J. H. (2010). Uranium distribution in the upper cretaceous-tertiary Belqa group. Yarmouk Valley, Northwest Jordan Jordan Journal of Earth and Environmental Sciences, 3, 49–62.
Peeters, L. (2014). A Background Color Scheme for Piper Plots to Spatially Visualize Hydrochemical Patterns Groundwater, 52, 2–6.
Rao, R., Satyanarayan, T., & Machiraju, P. (2012). Assessment of Ground Water Quality for Application in Kakinada Coast Der Chemica Sinica, 3, 287–291.
Rezaei, M., Nikbakht, M., & Shakeri, A. (2017). Geochemistry and sources of fluoride and nitrate contamination of groundwater in Lar area, south Iran. Environmental Science and Pollution Research, 24, 15471–15487.
Rimawi, O., & Al-Ansari, N. A. (1997). Groundwater degradation in the northeastern part of Mafraq area. Jordan IAHS Publications-Series of Proceedings and Reports-Intern Assoc Hydrological Sciences, 243, 235–244.
Sadashivaiah, C., Ramakrishnaiah, C., & Ranganna, G. (2008). Hydrochemical analysis and evaluation of groundwater quality in Tumkur Taluk. Karnataka State, India Int J Environ Res Public Health, 5, 158–164.
Sajo-Bohus, L., Gomez, J., Capote, T., Greaves, E., Herrera, O., Salazar, V., & Smith, A. (1997). Gross alpha radioactivity of drinking water in Venezuela. Journal of Environmental Radioactivity, 35, 305–312.
Salameh, E., & Udluft, P. (1985). The hydrodynamic pattern of the central part of Jordan. Geologisches Jahrbuch, 38, 39–53.
Sharma, T., Sharma, A., Kaur, I., Mahajan, R., Litoria, P., Sahoo, S., & Bajwa, B. (2019). Uranium distribution in groundwater and assessment of age dependent radiation dose in Amritsar. Gurdaspur and Pathankot Districts of Punjab, India Chemosphere, 219, 607–616.
Srilatha, M., Rangaswamy, D., & Sannappa, J. (2014). Studies on concentration of radon and physicochemical parameters in ground water around Ramanagara and Tumkur districts. Karnataka, India International Journal of Advanced Scientific and Technical Research, 2, 641–660.
Subramani, T., Elango, L., & Damodarasamy, S. (2005). Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin. Tamil Nadu, India Environ Geol, 47, 1099–1110.
Subramani, T., Rajmohan, N., & Elango, L. (2010). Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region. Southern India Environ Monit Assess, 162, 123–137.
USEPA (2018) DrinkingWater Standards and Health Advisories United States Environmental Protection Agency (USEPA), , washington DC
Tsiaili, A., Kiliari, T., & Pashalidis, I. (2011). Seasonal variation of the alpha-radioactivity concentration in natural water systems in Cyprus. Radiation Measurements, 46, 145–148.
Wu, Y., Li, J., Wang, Y., & Xie, X. (2018). Variations of uranium concentrations in a multi-aquifer system under the impact of surface water-groundwater interaction. Journal of Contaminant Hydrology, 211, 65–76.
WHO (2011) Guidelines for drinking-water quality vol 1. 3rd edn. World Health Organiszation,
Acknowledgements
The authors would like to thank the Water Authority of Jordan. All the measurements were conducted at isotopes and chemical analysis laboratories.
Disclosure statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alomari, A.H., Carvalho, F.P., Saleh, M.A. et al. Seasonal Variation In Major Ions Chemistry and Radionuclide Concentrations In Groundwater: A Case Study In The Amman-Zarqa Basin (Jordan). Water Air Soil Pollut 234, 256 (2023). https://doi.org/10.1007/s11270-023-06195-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06195-x