Abstract
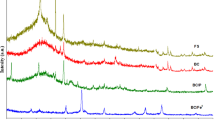
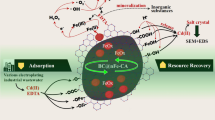
Methyl orange (MO) is a toxic dye used in many industrial processes. The removal of MO is considered expensive by the sophisticated treatment methods. Hence, there is a need for the discovery of novel methods for removing MO from polluted water. Therefore, this study was aimed at understanding the scientific insights into the adsorption mechanism of novel engineered biochar derived from neem chips via catalytic conversion with FeCl3 for the MO removal. This is the first report describing the use of novel engineered biochar derived from waste neem chip biomass for the removal of the highly resistant anionic dye “methyl orange” by understanding the insights into the removal mechanism. The biochar was prepared at different temperatures: 200 °C, 400 °C, 600 °C, and 800 °C, with a residence time of 2 h. An adsorptive experiment was planned, under a set of experimental conditions: dosage 1 g/L; pH 6; rpm 150; holding time 72 h, to identify the best biochar. The selected biochar has then been activated by iron catalyst of different concentrations (1%, 3%, 5%, and 7%) at a temperature of 700 °C for 30 min. The adsorptive performance was then checked. The engineered biochar with a high qe value (amount of adsorbate removed by unit weight of engineered biochar) was selected, for the detailed studies; isotherm, kinetics, thermodynamics, and rate-limiting factor analyses were used to understand the adsorptive mechanism of novel engineered biochar synthesized. Moreover, it was also treated with wastewater to check its removal efficiency. Furthermore, point zero charge (pzc) was analyzed to study the functional properties of novel engineered biochar along with XRD analysis. The outcomes revealed that the FeCl3 activation improved the (qe) amount of adsorbate removed by grams of engineered biochar to 63.39 mg/g from 80.30 mg/g and it improved the aromatic carbon network. Moreover, the adsorption nature of novel engineered biochar to MO removal is multilayer. It also obeyed well for the pseudo-second-order kinetics. The adsorption is spontaneous and endothermic. The engineered biochar synthesized performed well to remove impurities from a real industrial wastewater: Removal of total suspended solids was 68% and removal of total solids was 74%. Therefore, the engineered biochar produced by the iron catalytic conversion of neem chips is a novel adsorbent for MO removal from aqueous phase.
Graphical Abstract

Highlights
• Novel engineered biochar from neem chips has the potential to remove industrial dyes.
• Pyrolysis temperature had a significant influence on the performance of the biochar.
• FeCl3 activation of neem chips enhances the adsorption of methyl orange by two-fold.
• Surface functional properties of the biochar were improved by iron catalyst activation.













Similar content being viewed by others
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Agarwal, A., & Vaishali. (2016). Removal of methyl orange dye from textile effluent using adsorption on chitosan hydrogel beads. ESSENCE - International Journal for Environmental Rehabilitation and Conservation, VI, I(2), 73–80.
Ahmed Jasim, M. & Mohammed Abbas, A. (2019). Adsorption of malachite green dye by biomicro-adsorbent from aqueous solution at different temperatures. Journal of Physics: Conference Series, 1294(5). https://doi.org/10.1088/1742-6596/1294/5/052015
Al-wabel, M. I., Al-omran, A., El-naggar, A. H., Nadeem, M., & Usman, A. R. A. (2013). Bioresource technology pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from Conocarpus wastes. Bioresource Technology, 131, 374–379. https://doi.org/10.1016/j.biortech.2012.12.165
Bazrafshan, E., Zarei, A. A., Nadi, H., & Zazouli, M. A. (2014). Adsorptive removal of methyl 827 orange and reactive red 198 dyes by Moringa peregrina ash. Indian Journal of Chemical Technology, 21(2), 105–113.
Berwal, N., Kundu, R. S., Nanda, K., Punia, R., & Kishore, N. (2015). Physical, structural and optical characterizations of borate modified bismuth–silicate–tellurite glasses. Journal of Molecular Structure, 1097, 37–44.
Bhattacharyya, K. G., & Sharma, A. (2005). Kinetics and thermodynamics of methylene blue adsorption on neem (Azadirachta indica) leaf powder. https://doi.org/10.1016/j.dyepig.2004.06.016
Block, I., Günter, C., Rodrigues, A. D., Paasch, S., Hesemann, P., & Taubert, A. (2021). Carbon adsorbents from spent coffee for removal of methylene blue and methyl orange from water. Materials, 14(14). https://doi.org/10.3390/ma14143996
Bonelli, P. R., Della Rocca, P. A., Cerrella, E. G., & Cukierman, A. L. (2001). Effect of pyrolysis temperature on composition, surface properties and thermal degradation rates of Brazil nut shells. Bioresource Technology, 76(1), 15–22. https://doi.org/10.1016/S09608524(00)00085-7
Campos, N. F., Barbosa, C. M. B. M., Rodríguez-Díaz, J. M., & Duarte, M. M. M. B. (2018). Removal of naphthenic acids using activated charcoal: Kinetic and equilibrium studies. Adsorption Science and Technology, 36(7–8), 1405–1421. https://doi.org/10.1177/0263617418773844
Chai, W. S., Cheun, J. Y., Kumar, P. S., Mubashir, M., Majeed, Z., Banat, F., Ho, S. H., & Show, P. L. (2021). A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. Journal of Cleaner Production, 296, 126589. https://doi.org/10.1016/j.jclepro.2021.126589
Farooq, M., & Ramli, A. (2011). The determination of point zero charge (PZC) of Al 2O 3-MgO 847 mixed oxides. 2011 National Postgraduate Conference - Energy and Sustainability: Exploring the Innovative Minds, NPC 2011, 0–4. https://doi.org/10.1109/NatPC.2011.6136447
Garg, V. K., Gupta, R., Yadav, A. B., & Kumar, R. (2003). Dye removal from aqueous solution by adsorption on treated sawdust. Bioresource Technology, 89(2), 121–124. https://doi.org/10.1016/S0960-8524(03)00058-0
Godlewska, P., Bogusz, A., Dobrzyńska, J., Dobrowolski, R., & Oleszczuk, P. (2020). Engineered biochar modified with iron as a new adsorbent for treatment of water contaminated by selenium. Journal of Saudi Chemical Society, 24(11), 824–834. https://doi.org/10.1016/j.jscs.2020.07.006
Gökkuş, Ö., & Oğuz, M. (2011). Investigation of color and cod removal by Fenton reagent from aqueous solutions containing acid and reactive dyestuffs. Desalination and Water Treatment, 26(1–3), 160–164. https://doi.org/10.5004/dwt.2011.2119
Gökkuş, Ö., Çoşkun, F., Kocaoğlu, M., & Yıldız, Y. Ş. (2014). Determination of optimum conditions for color and COD removal of Reactive Blue 19 by Fenton oxidation process. Desalination and Water Treatment, 52(31–33), 6156–6165. https://doi.org/10.1080/19443994.2013.812523
Hamzezadeh, A., Rashtbari, Y., Afshin, S., Morovati, M., & Vosoughi, M. (2022). Application of low-cost material for adsorption of dye from aqueous solution. International Journal of Environmental Analytical Chemistry, 102(1), 254–269. https://doi.org/10.1080/03067319.2020.1720011
He, W., Ma, Q., Wang, J., Yu, J., Bao, W., Ma, H., & Amrane, A. (2014). Preparation of novel kaolin-based particle electrodes for treating methyl orange wastewater. Applied Clay Science, 99, 178–186. https://doi.org/10.1016/j.clay.2014.06.030
Hii, H. T. (2021). Adsorption isotherm and kinetic models for removal of methyl orang and remazol brilliant blue r by coconut shell activated carbon. Tropical Aquatic and Soil Pollution, 1(1), 1–10. https://doi.org/10.53623/tasp.v1i1.4
Iyyapushpam, S., Nishanthi, S. T., & Padiyan, D. P. (2013). Photocatalytic degradation of methyl orange using a -Bi 2 O 3 prepared without surfactant. Journal of Alloys and Compounds, 563, 104–107. https://doi.org/10.1016/j.jallcom.2013.02.107
Kloss, S., Zehetner, F., Dellantonio, A., Hamid, R., Ottner, F., Liedtke, V., Schwanninger, M., Gerzabek, M. H., & Soja, G. (2012). Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. Journal of Environmental Quality, 41(4), 990–1000. https://doi.org/10.2134/jeq2011.0070
Kumar, N. S., Shaikh, H. M., Asif, M., & Al-Ghurabi, E. H. (2021). Engineered biochar from wood apple shell waste for high-efficient removal of toxic phenolic compounds in wastewater. Scientific Reports, 11(1), 1–17. https://doi.org/10.1038/s41598-021-82277-2
Lonappan, L., Rouissi, T., Brar, S. K., Verma, M., & Surampalli, R. Y. (2018). Adsorption of diclofenac onto different biochar microparticles: Dataset – Characterization and dosage of biochar. Data in Brief, 16, 460–465. https://doi.org/10.1016/j.dib.2017.10.041
Manoharan, T., Ganeshalingam, S., & Nadarajah, K. (2022). Mechanisms of emerging contaminants removal by novel neem chip biochar. Environmental Advances, 7, 100158. https://doi.org/10.1016/j.envadv.2021.100158
Mohammadi, N., Khani, H., Gupta, V. K., Amereh, E., & Agarwal, S. (2011). Adsorption process of methyl orange dye onto mesoporous carbon material-kinetic and thermodynamic studies. Journal of Colloid and Interface Science, 362(2), 457–462. https://doi.org/10.1016/j.jcis.2011.06.067
Nadarajah, K., Bandala, E. R., Zhang, Z., Mundree, S., & Goonetilleke, A. (2021). Removal of 881 heavy metals from water using engineered hydrochar: Kinetics and mechanistic approach. Journal of Water Process Engineering, 40(January), 101929. https://doi.org/10.1016/j.jwpe.2021.101929
Namasivayam, C., & Kavitha, D. (2006). IR, XRD and SEM studies on the mechanism of adsorption of dyes and phenols by coir pith carbon from aqueous phase. Microchemical Journal, 82(1), 43–48. https://doi.org/10.1016/j.microc.2005.07.002
Nguyen, L. H., Ngo, Q. N., Van, H. T., Thai, V. N., Nguyen, T. P., & PhanThi, K. O. (2021). Reutilization of Fe-containing tailings ore enriched by iron(III) chloride as a heterogeneous Fenton catalyst for decolorization of organic dyes. RSC Advances, 11(26), 15871–15884. https://doi.org/10.1039/d1ra02939h
Pal, J., Deb, M. K., Deshmukh, D. K., & Verma, D. (2013). Removal of methyl orange by activated 892 carbon modified by silver nanoparticles. Applied Water Science, 3(2), 367–374. https://doi.org/10.1007/s13201-013-0087-0
Park, H., Kim, J., & Lee, Y. (2021). Enhanced Adsorptive removal of dyes using mandarin peel biochars via Chemical Activation with NH 4 Cl and ZnCl 2.
Sajjadi, B., Chen, W. Y., & Egiebor, N. O. (2019). A comprehensive review on physical activation of biochar for energy and environmental applications. Reviews in Chemical Engineering, 35(6), 735–776. https://doi.org/10.1515/revce-2017-0113
Samar K. Theydan, M. J. A. (n.d.). Adsorption of methylene blue onto biomass-based activated carbon by FeCl3 activation: Equilibrium, kinetics, and thermodynamic studies. Journal of Analytical and Applied Pyrolysis. https://doi.org/10.1016/j.jaap.2012.05.008
Soldatkina, L., & Zavrichko, M. (2019). Equilibrium, kinetic, and thermodynamic studies of anionic dyes adsorption on corn stalks modified by cetylpyridinium bromide. Colloids and Interfaces, 3(1). https://doi.org/10.3390/colloids3010004
Song, M., Jin, B., Xiao, R., Yang, L., Wu, Y., Zhong, Z., & Huang, Y. (2013). The comparison of two activation techniques to prepare activated carbon from corn cob. Biomass and Bioenergy, 48, 250–256. https://doi.org/10.1016/j.biombioe.2012.11.007
Soylu, M., Gökkuş, Ö., & Özyonar, F. (2020). Foam separation for effective removal of disperse and reactive dyes from aqueous solutions. Separation and Purification Technology, 247(December 2019). https://doi.org/10.1016/j.seppur.2020.116985
Wasewar, K. L., Prasad, B., & Gulipalli, S. (2009). Removal of selenium by adsorption onto granular activated carbon (GAC) and powdered activated carbon (PAC). Clean - Soil, Air, Water, 37(11), 872–883. https://doi.org/10.1002/clen.200900188
Yönten, V., Sanyürek, N. K., & Kivanç, M. R. (2020). A thermodynamic and kinetic approach to adsorption of methyl orange from aqueous solution using a low cost activated carbon prepared from Vitis vinifera L. Surfaces and Interfaces, 20, 100529. https://doi.org/10.1016/j.surfin.2020.100529
Zhang, Z., Moghaddam, L., O’Hara, I. M., & Doherty, W. O. S. (2011). Congo Red adsorption by ball-milled sugarcane bagasse. Chemical Engineering Journal, 178, 122–128. https://doi.org/10.1016/j.cej.2011.10.024
Zhu, C., Wang, L., Kong, L., Yang, X., Wang, L., Zheng, S., Chen, F., Maizhi, F., & Zong, H. (2000). Photocatalytic degradation of AZO dyes by supported TiO22 + UV in aqueous solution. Chemosphere, 41(3), 303–309. https://doi.org/10.1016/S0045-6535(99)00487-7
Acknowledgements
The authors gratefully acknowledge the support from Department of Agricultural Engineering, Faculty of Agriculture, University of Jaffna, Sri Lanka, to undertake this study. They would also like to thank Department of Civil Engineering, Faculty of Engineering, University of Jaffna, Sri Lanka, for instrumental support. Moreover, they thank Dr. M. Thanihaichelvan, Department of Physics, Faculty of Science, University of Jaffna, Sri Lanka, for the technical support to perform XRD analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sunthar, L., Asharp, T. & Nadarajah, K. Insights into Mechanisms of Novel Engineered Biochar Derived from Neem Chips via Iron Catalyst for the Removal of Methyl Orange from Aqueous Phase . Water Air Soil Pollut 234, 178 (2023). https://doi.org/10.1007/s11270-023-06187-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06187-x