Abstract
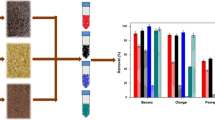
Dyes are the main contaminant from the textile industry wastes. Dyes can severely harm the environment, disturbing all the water cycle and organisms living in these environments. The juice industry generates waste of the peel from a great number of fruits. The peel of Passiflora edulis is rich in pectin, a polymer with great adsorption potential for textile dye sorption. However, the lack of applicability and adsorption interaction knowledge of the use of pectin as textile dye adsorbent makes it impossible to apply them, being necessary more studies with different dyes for its application and understanding of the chemical interactions of adsorbent/adsorbate. Thus, the aim of the present study was to evaluate the adsorptive potential of pectin present in the peel residue of P. edulis. Adsorption experiments were carried out to analyze the kinetics, isotherm, and thermodynamics, seeking the optimization of the adsorption treatment. Scanning electron microscopy and Fourier transform infrared spectrophotometry were used to characterize and analyze the main adsorption sites of the pectin from P. edulis peels. Bioassays using the Lactuca sativa seeds confirmed the reduction of the toxicity of the solution after the adsorption treatment. The results also confirmed a chemisorption occurrence, confirming the strong interaction between the pectin and the textile dye. Therefore, the material tested in the present study can potentially be applied for textile waste treatment, removing dyes from effluents, in addition to giving a noble destination for a residue from the juice industry.











Similar content being viewed by others
Data Availability
All the data produced in this work are stored at the online drive server from the research group. The server is not public, but when requested the link and key access will be shared for the access.
References
Adane, B., Siraj, K., & Meka, N. (2015). Kinetic, equilibrium and thermodynamic study of 2-chlorophenol adsorption onto Ricinus communis pericarp activated carbon from aqueous solutions. Green Chemistry Letters and Reviews. https://doi.org/10.1080/17518253.2015.1065348
Ahmad, A. A., Idris, A., & Hameed, B. H. (2013). Organic dye adsorption on activated carbon derived from solid waste. Desalination and Water Treatment. https://doi.org/10.1080/19443994.2012.749019
Almeida, E. J. R., & Corso, C. R. (2014). Comparative study of toxicity of azo dye Procion Red MX-5B following biosorption and biodegradation treatments with the fungi Aspergillus niger and Aspergillus terreus. Chemosphere, 112, 317–322.
Balarak, D., Zafariyan, M., Igwegbe, C. A., Onyechi, K. K., & Ighalo, J. O. (2021). Adsorption of Acid Blue 92 dye from aqueous solutions by single-walled carbon nanotubes: Isothermal, kinetic, and thermodynamic studies. Environmental Processes, 8, 869–888.
Boyd, G. E., Adamson, A. W., & Myers, L. S. (1947). The exchange adsorption of ions from aqueous solution by organic zeolites Kinetics. Journal of the American Chemical Society, 69, 2836–2848.
Chatterjee, S., Guha, N., Krishnan, S., Singh, A. K., Mathur, P., & Rai, D. K. (2020). Selective and recyclable Congo Red dye adsorption by spherical Fe3O4 nanoparticles functionalized with 1,2,4,5-benzenetetracarboxylic acid. Scientific Reports. https://doi.org/10.1038/s41598-019-57017-2
Chen, K., Du, L., Gao, P., Zheng, J., Liu, Y., & Lin, H. (2021). Super and selective adsorption of cationic dyes onto carboxylate-modified passion fruit peel biosorbent. Frontiers in Chemistry. https://doi.org/10.3389/fchem.2021.646492
Coelho, E. M., Azevedo, L. C., Viana, A. C., Ramos, I. G., Gomes, R. G., Lima, M. S., & Umsza-Guez, M. A. (2018). Physico-chemical properties, rheology and degree of esterification of passion fruit (Passiflora edulis f. flavicarpa) peel flour. Journal of the Science of Food and Agriculture, 98, 166–173.
Crini, G., & Badot, P. M. (2008). Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solution by adsorption process using batch studies. Journal Progress in Polymer Science, 33, 399–447.
Dilarri, G., Almeida, E. J. R., Pecora, H. B., & Corso, C. R. (2016). Removal of dye toxicity from an aqueous solution using an industrial strain of Saccharomyces cerevisiae (Meyen). Water, Air and Soil Pollution, 227, 269–283.
Dilarri, G., Montagnolli, R. N., Bidoia, E. D., Mendes, C. R., & Corso, C. R. (2018). Kinetic, isothermal, and thermodynamic models to evaluate Acid Blue 161 dye removal using industrial chitosan powder. Desalination and Water Treatment, 109, 261–270.
Elkady, M., Shokry, H., El-Sharkawy, A., El-Subruiti, G., & Hamad, H. (2019). New insights into the activity of green supported nanoscale zero-valent iron composites for enhanced acid blue-25 dye synergistic decolorization from aqueous medium. Journal of Molecular Liquids. https://doi.org/10.1016/j.molliq.2019.111628
Fat’hi, M. R., Asfaram, A., Hadipour, A., & Roosta, M. (2014). Kinetics and thermodynamic studies for removal of acid blue 129 from aqueous solution by almond shell. Journal of Environmental Health Science & Engineering, 12, 62.
Fayazi, M., Ghanei-Motlagh, M., & Taher, M. A. (2015). The adsorption of basic dye (Alizarin red S) from aqueous solution onto activated carbon/γ-Fe2O3 nano-composite: Kinetic and equilibrium studies. Materials Science in Semiconductor Processing, 40, 35–43.
Foo, K. Y., & Hameed, B. H. (2010). An overview of dye removal via activated carbon adsorption process. Desalination and Water Treatment, 19, 255–274.
Freundlich, H. (1906). Adsorption in solution. Journal of Physical Chemistry, 40, 1361–1368.
Gerola, G. P., Boas, N. V., Caetano, J., Tarley, C. R. T., Goncalves, A. C., Jr., & Dragunski, D. C. (2013). Utilization of passion fruit skin by-product as lead(II) ion biosorbent. Water, Air, & Soil Pollution, 224, 1446.
Gouthaman, A., Azarudeen, R. S., Gnanaprakasam, A., Sivakumar, V. M., & Thirumarimurugan, M. (2018). Polymeric nanocomposites for the removal of Acid red 52 dye from aqueous solutions: Synthesis, characterization, kinetic and isotherm studies. Ecotoxicology and Environmental Safety, 160, 42–51.
Hatami, T., Santos, L. C., Zabot, G. L., Pontes, P. V. A., Batista, E. A. C., Mei, L. H. I., & Martínez, J. (2020). Integrated supercritical extraction and supercritical adsorption processes from passion fruit by-product: Experimental and economic analyses. Journal of Supercritical Fluids. https://doi.org/10.1016/j.supflu.2020.104856
Ho, Y. S., & McKay, G. (1998). Sorption of dye from aqueous solution by peat. Chemical Engineering Journal, 70, 115–124.
Jawad, A. H., & Abdulhameed, A. S. (2020). Statistical modeling of methylene blue dye adsorption by high surface area mesoporous activated carbon from bamboo chip using KOH-assisted thermal activation. Energy, Ecology and Environment, 5, 456–469.
Lagergren, S. (1898). The theory of adsorption of substances. Handlingar, 24, 1–39.
Langmuir, I. (1918). The adsorption of gases on plane surface of glass, mica and platinum. Journal of the American Chemical Society, 40, 1361–1368.
Lin, H., Chen, K., Du, L., Gao, P., Zheng, J., Liu, Y., & Ma, L. (2021). Efficient and selective adsorption of methylene blue and methyl violet dyes by yellow passion fruit peel. Environmental Technology. https://doi.org/10.1080/09593330.2021.1924288
Liu, Y., Ying, D., Sanguansri, L., & Augustin, M. A. (2019). Comparison of the adsorption behaviour of catechin onto cellulose and pectin. Food Chemistry, 271, 733–738.
Mekki, A., Dhouib, A., & Sayadi, S. (2007). Polyphenols dynamics and phytotoxicity in a soil amended by olive mill wastewaters. Journal of Environmental Management, 84, 134–140.
Mendes, C. R., Dilarri, G., Stadioto, M. R., Faria, A. U., Bidoia, E. D., & Montagnolli, R. N. (2019). The addition of a quaternary group in biopolymeric material increases the adsorptive capacity of Acid Blue 25 textile dye. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-019-05652-7
Mirza, A., & Ahmad, R. (2020). An efficient sequestration of toxic crystal violet dye from aqueous solution by Alginate/Pectin nanocomposite: A novel and ecofriendly adsorbent. Groundwater for Sustainable Development, 11, 100373.
Moraes, J. R., Jr., & Bidoia, E. D. (2015). Colour degradation of simulated textile effluent by electrolytic treatment and ecotoxicology evaluation. Water, Air, & Soil Pollution, 226, 402.
Moura, F. A., Macagnan, F. T., Petkowicz, C. L. O., & Silva, L. P. (2020). Partially hydrolyzed pectin extracted from passion fruit peel: Molar mass and physicochemical properties. Bioactive Carbohydrates and Dietary Fibre. https://doi.org/10.1016/j.bcdf.2019.100206
Muntean, M. C., Farcas, A. C., Medeleanu, M., Salanta, L. C., & Borsa, A. (2022). A sustainable approach for the development of innovative products from fruit and vegetable by-products. Sustainability. https://doi.org/10.3390/su141710862
Nayak, A. K., & Pal, A. (2020). Statistical modeling and performance evaluation of biosorptive removal of Nile Blue A by lignocellulosic agricultural waste under the application of high-strength dye concentrations. Journal of Environmental Chemical Engineering. https://doi.org/10.1016/j.jece.2020.103677
Nouri, L., Hemidouche, S., Boudjemaa, A., Kaouah, F., Sadaoui, Z., & Bachari, K. (2020). Elaboration and characterization of photobiocomposite beads, based on titanium (IV) oxide and sodium alginate biopolymer, for basic blue 41 adsorption/photocatalytic degradation. International Journal of Biological Macromolecules, 151, 66–84.
Pauletto, P. S., Gonçalves, J. O., Pinto, L. A. A., Dotto, G. L., & Salau, N. P. G. (2020). Single and competitive dye adsorption onto chitosan–based hybrid hydrogels using artificial neural network modeling. Journal of Colloid and Interface Science, 560, 722–729.
Pecora, H. B., Dilarri, G., Mendes, C. R., & Corso, C. R. (2018). Bioassays and coagulation studies using Moringa oleifera seeds for the removal of textile dyes. Water Science and Technology, 78, 1679–1692.
Ramos, B. P., Perez, I. D., Paiano, M. S., Vieira, M. G. A., & Boina, R. F. (2022). Activated carbons from passion fruit shells in adsorption of multimetal wastewater. Environmental Science and Pollution Research, 29, 1446–1457.
Ranganathan, P., Mutharani, B., Chen, S. M., & Sireesha, P. (2019). Biocompatible chitosan-pectin polyelectrolyte complex for simultaneous electrochemical determination of metronidazole and metribuzin. Carbohydrate Polymers, 214, 317–327.
Saber-Samandari, S., & Heydaripour, J. (2015). Onion membrane: An efficient adsorbent for decoloring of wastewater. Journal of Environmental Health Science & Engineering, 13, 16.
Siddiqui, H. Z., & Abbas, Z. K. (2021). Assessment of phytotoxicity of treated water of Tabuk wastewater plant by different technologies on seed germination of chick pea (Cicer arietinum). Water Science and Technology, 84, 2968–2979.
Subbareddy, Y., Kumar, R. N., Sudhakar, B. K., Reddy, K. R., Martha, S. K., & Kaviyarasu, K. (2020). A facile approach of adsorption of acid blue 9 on aluminium silicate-coated Fuller’s Earth––Equilibrium and kinetics studies. Surfaces and Interfaces. https://doi.org/10.1016/j.surfin.2020.100503
Thinakaran, N., Panneerselvam, P., Baskaralingam, P., Elango, D., & Sivanesan, S. (2009). Equilibrium and kinetic studies on the removal of Acid Red 114 from aqueous solutions using activated carbons prepared from seed shells. Journal of Hazardous Materials, 158, 142–150.
Van’t Hoff, J. H. (1874). A suggestion looking to the extension into space of the structural formulas at present used in chemistry. And a note upon the relation between the optical activity and the chemical constitution of organic compounds. Archives Neerlandaises des Sciences Exactes et Naturelles, 9, 445-454.
Wang, R., Liang, R., Dai, T., Chen, J., Shuai, X., & Liu, C. (2019). Pectin-based adsorbents for heavy metal ions: A review. Trends in Food Science & Technology. https://doi.org/10.1016/j.tifs.2019.07.033
Weber, W. J., & Morris, J. C. (1963). Kinetics of adsorption on carbon from solution. Journal of the Sanitary Engineering Division, 89, 31–60.
Acknowledgements
Carolina Rosai Mendes received a PhD scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil.
Funding
This work was funded by the National Council for Scientific and Technological Development (CNPq)—process: 150092/2022–9.
Author information
Authors and Affiliations
Contributions
C.R.M. and G.D.: conceptualization, methodology, experiments (adsorption, bioassays, and FT-IR analysis), validation, formal analysis, writing original draft; M.R.S.: SEM analysis; E.D.B. and R.N.M.: conceptualization, writing/review and editing, supervision, funding acquisition, project administration.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mendes, C.R., Dilarri, G., Stradioto, M.R. et al. Sustainable Waste Recycling from the Fruit Pulp Industry Applied as an Adsorbent of Textile Dye. Water Air Soil Pollut 233, 487 (2022). https://doi.org/10.1007/s11270-022-05964-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05964-4