Abstract
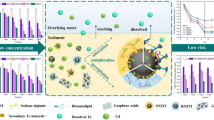
In view of the increasingly prominent heavy metal pollution problem in water, D201 resin-supported polyethylene glycol–modified nanoscale zero-valent iron (PEG-nZVI/D201) was fabricated to remove Cr(VI) in wastewater. PEG-nZVI/D201 can overcome the agglomeration and oxidation drawbacks of nZVI effectively, and improve the removal efficiency of Cr(VI). Its Cr(VI) removal rate was 33.6% higher than that of bare nZVI when the dosage of PEG-nZVI/D201 was 1.0 g·L−1, the Cr(VI) concentration was 50 mg·L−1, and the initial pH was 5.0. The maximum adsorption capacity of Cr(VI) on PEG-nZVI/D201 reached 134.56 mg·g−1 at 318 K. The Langmuir model better describes the adsorption of Cr(VI) on PEG-nZVI/D201. Thermodynamic parameters show that the adsorption process is spontaneous and endothermic. The mechanism of Cr(VI) removal by PEG-nZVI/D201 is as follows: firstly, protonated PEG-nZVI/D201 adsorbs Cr(VI) by electrostatic attraction, then nZVI reduces most of Cr(VI) to Cr(III) through its strong reducibility, and finally, Cr(III) can form CrXFe1-X(OH)3 co-precipitation with Fe(III) produced by oxidation of nZVI.









Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Chen, N., et al. (2012). Preparation and characterization of lanthanum(III) loaded granular ceramic for phosphorus adsorption from aqueous solution. Journal of the Taiwan Institute of Chemical Engineers, 43(5), 783–789.
Costa, M., et al. (2006). Toxicity and carcinogenicity of chromium compounds in humans. Critical Reviews in Toxicology, 36(2), 155–163.
Elwakeel, K. Z., et al. (2020). Microwave assist sorption of crystal violet and Congo red dyes onto amphoteric sorbent based on upcycled Sepia shells. Journal of Environmental Health Science and Engineering, 18, 35–50.
Enzo et al., (2019). Preparation of quaternary amine-grafted organosolv lignin biosorbent and its application in the treatment of hexavalent chromium polluted water. International Journal of Biological Macromolecules, 126:1014-1022
Fan, Z., et al. (2019). Removal of hexavalent chromium by biochar supported nZVI composite: Batch and fixed-bed column evaluations, mechanisms, and secondary contamination prevention. Chemosphere, 217, 85–94.
Fan, H. L., et al. (2020). High-gravity continuous preparation of chitosan-stabilized nanoscale zero-valent iron towards Cr(VI) removal. Chemical Engineering Journal, 390, 124639.
Fu, B. L., et al. (2013). Chromium removal using resin supported nanoscale zero-valent iron. Journal of Environmental Management, 128, 822–827.
Fu, R. B., et al. (2015). The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere, 138, 726–734.
Fu, R. B., et al. (2017). Fast and highly efficient removal of chromium (VI) using humus-supported nanoscale zero-valent iron: Influencing factors, kinetics and mechanism. Separation and Purification Technology, 174, 362–371.
Gao, W., et al. (2020). Nano zero-valent iron supported by macroporous styrene ion exchange resin for enhanced Cr(VI) removal from aqueous solution. Journal of Dispersion Science and Technology, 43, 1197–1207.
Guo, J., et al. (2020). Synthesis, Cr(VI) removal performance and mechanism of nanoscale zero-valent iron modified potassium-doped graphitic carbon nitride. Water Science and Technology, 81(9), 1840–1851.
Hamdy, A., et al. (2020). Characterization of superparamagnetic/monodisperse PEG-coated magnetite nanoparticles sonochemically prepared from the hematite ore for Cd(II) removal from aqueous solutions. Journal of Inorganic and Organometallic Polymers and Materials, 31(1), 397–414.
Hedayatkhah, A., et al. (2018). Bioremediation of chromium contaminated water by diatoms with concomitant lipid accumulation for biofuel production. Journal of Environmental Management, 227, 313–320.
Ji, B., et al. (2018). Chromium (VI) removal from water using starch coated nanoscale zerovalent iron particles supported on activated carbon. Chemical Engineering Communications, 206(6), 708–715.
Kera, N. H., et al. (2016). Selective removal of Cr(VI) from aqueous solution by polypyrrole/2,5-diaminobenzene sulfonic acid composite. Journal of Colloid and Interface Science, 476, 144–157.
Lalley, J., et al. (2016). Phosphate adsorption using modified iron oxide-based sorbents in lake water: Kinetics, equilibrium, and column tests. Chemical Engineering Journal, 284, 1386–1396.
Li, X. Q., et al. (2008). Stoichiometry of Cr(VI) Immobilization using nanoscale zerovalent iron (NZVI): A study with high-resolution X-ray photoelectron spectroscopy (HR-XPS). Industrial & Engineering Chemistry Research, 47, 2131–2139.
Li, Q., et al. (2010). Equilibrium, thermodynamics and process design to minimize adsorbent amount for the adsorption of acid dyes onto cationic polymer-loaded bentonite. Chemical Engineering Journal, 158, 489–497.
Li, X. Y., et al. (2016). Nanoscale zerovalent iron decorated on graphene nanosheets for Cr(VI) removal from aqueous solution: Surface corrosion retard induced the enhanced performance. Chemical Engineering Journal, 288, 789–797.
Liu, X., et al. (2021). Orderly porous covalent organic frameworks-based materials: Superior adsorbents for pollutants removal from aqueous solutions. Innovation, 2(1), 100076.
Lo, I. M. C., et al. (2006). Hardness and carbonate effects on the reactivity of zero-valent iron for Cr(VI) removal. Water Research, 40, 595–605.
Lv, X. S., et al. (2011). Removal of chromium(VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere, 85, 1204–1209.
Lv, X. S., et al. (2012). Highly active nanoscale zero-valent iron (nZVI)-Fe3O4 nanocomposites for the removal of chromium(VI) from aqueous solutions. Journal of Colloid and Interface Science, 369, 460–469.
Lv, X. S., et al. (2014). Nanoscale zero-valent iron (nZVI) assembled on magnetic Fe3O4/graphene for chromium (VI) removal from aqueous solution. Journal of Colloid and Interface Science, 417, 51–59.
Madan, S., et al. (2020). Enhancing corrosion stability and shelf life of nanoscale zero-valent iron via encapsulation in porous Ze-TiO2 matrix: An interface for simultaneous oxidation and adsorption of As(III). Colloids and Surfaces a: Physicochemical and Engineering Aspects, 607, 125381.
O’Carroll, D., et al. (2013). Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Advances in Water Resources, 51, 104–122.
Owlad, M., et al. (2009). Removal of hexavalent chromium-contaminated water and wastewater: A review. Water Air and Soil Pollution, 200, 59–77.
Peng, Z. L., et al. (2017). Facile modification of nanoscale zero-valent iron with high stability for Cr(VI) remediation. Science of the Total Environment, 596, 266–273.
Rivero-Huguet, M., et al. (2009). Reduction of hexavalent chromium mediated by micron- and nano-scale zero-valent metallic particles. Journal of Environmental Monitoring, 11(5), 1072–1079.
Sarathy, V., et al. (2008). Aging of iron nanoparticles in aqueous solution. Journal of Physical Chemistry C, 112(7), 2286–2293.
Shi, L. N., et al. (2011). Removal of chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Research, 45, 886–892.
Singh, R., et al. (2012). Removal of hexavalent chromium from contaminated ground water using zero-valent iron nanoparticles. Environmental Monitoring and Assessment, 184, 3643–3651.
Singh, P., et al. (2014). Solar-Fenton removal of malachite green with novel Fe0-activated carbon nanocomposite. Applied Catalysis a: General, 476, 9–18.
Son, E. B., et al. (2018). Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Science of the Total Environment, 615, 161–168.
Song, Y., et al. (2020). Removal of trace Cr(VI) from aqueous solution by porous activated carbon balls supported by nanoscale zero-valent iron composites. Environmental Science and Pollution Research, 27, 7015–7024.
Stefaniuk, M., et al. (2016). Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chemical Engineering Journal, 287, 618–632.
Sun, X. T., et al. (2014). Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr(VI): Synthesis and adsorption studies. Chemical Engineering Journal, 241, 175–183.
Theron, J., et al. (2008). Nanotechnology and water treatment: Applications and emerging. Critical Reviews in Microbiology, 34, 43–69.
Tian, H., et al. (2018). Surfactant-enhanced PEG-4000-NZVI for remediating trichloroethylene-contaminated soil. Chemosphere, 195, 585–593.
Vaiopoulou, E., et al. (2012). Effects of chromium on activated sludge and on the performance of wastewater treatment plants: A review. Water Research, 46, 549–570.
Wang, X. Y., et al. (2017a). A biomimetic strategy for improving activity of nano zero-valent iron based on the adhesive behavior of polydopamine on PVDF·Al2O3 film. Materials Chemistry and Physics, 197, 215–225.
Wang, J. G., et al. (2017). Efficient removal of fluoride using polypyrrole-modified biochar derived from slow pyrolysis of pomelo peel: Sorption capacity and mechanism. Journal of Polymers and the Environment, 26(4), 1559–1572.
Wang, H. H., et al. (2019). Enhanced photoreduction of U(VI) on C3N4 by Cr(VI) and bisphenol A: ESR, XPS, and EXAFS investigation. Environmental Science and Technology, 53(11), 6454–6461.
Wang, J. H., et al. (2020). Adsorption behavior and mechanism of aqueous Cr(III) and Cr(III)-EDTA chelates on DTPA-chitosan modified Fe3O4@SiO2. Reactive and Functional Polymers, 156, 104720.
Wang, S., et al. (2021). Polyethylene glycol-stabilized bimetallic nickel–zero valent iron nanoparticles for efficient removal of Cr(VI). New Journal of Chemistry, 45, 13969–13978.
Wen, R. T., et al. (2019). An ion release controlled Cr(VI) treatment agent: Nano zero-valent iron/carbon/alginate composite gel. International Journal of Biological Macromolecules, 146, 692–704.
Wu, H., et al. (2020). Polyethylene glycol-stabilized nano zero-valent iron supported by biochar for highly efficient removal of Cr(VI). Ecotoxicology and Environmental Safety, 188, 109902.
Xiong, Y., et al. (2019). Improved removal capacity of magnetite for Cr(VI) by electrochemical reduction. Journal of Hazardous Materials, 374, 26–34.
Yao, L., et al. (2013). N-doped porous carbon with magnetic particles formed in situ for enhanced Cr(VI) removal. Water Research, 47, 4188–4197.
Yoshinaga, M., et al. (2018). A comprehensive study including monitoring, assessment of health effects and development of a remediation method for chromium pollution. Chemosphere, 201, 667–675.
Zeng, L., et al. (2004). Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings. Water Research, 38, 1318–1326.
Zhang, Y. Y., et al. (2013). The dispersity-dependent interaction between montmorillonite supported nZVI and Cr(VI) in aqueous solution. Chemical Engineering Journal, 229, 412–419.
Zhang, M. M., et al. (2015). Chitosan modification of magnetic biochar produced from Eichhornia crassipes for enhanced sorption of Cr(VI) from aqueous solution. RSC Advances, 5(58), 46955–469640.
Zhang, S., et al. (2019). A novel biochar supported CMC stabilized nano zero-valent iron composite for hexavalent chromium removal from water. Chemosphere, 217, 686–694.
Zhao, Y. G., et al. (2010). Preparation and characterization of amino-functionalized nano-Fe3O4 magnetic polymer adsorbents for removal of chromium(VI) ions. Journal of Materials Science, 45, 5291–5301.
Zhao, S. F., et al. (2020). Enhanced removal of Cr(VI) from wastewater by nanoscale zero valent iron supported on layered double hydroxides. Journal of Porous Materials, 27(6), 1701–1710.
Zhou, H., et al. (2008). Influence of complex reagents on removal of chromium(VI) by zero-valent iron. Chemosphere, 72, 870–874.
Zhou, H. Y., et al. (2019). Mechanism and influence factors of 2,4-D dechlorination by sodium citrate-activated bimetallic palladium-zero valent iron nanoparticles. Applied Organometallic Chemistry, 34, 5324.
Zhu, Y., et al. (2019a). Mechanisms of Cr(VI) reduction by Bacillus sp. CRB-1, a novel Cr(VI)-reducing bacterium isolated from tannery activated sludge. Ecotoxicology and Environmental Safety, 186, 109792.
Zhu, Y., et al. (2019b). Effects of surface-modified biochars and activated carbon on the transformation of soil inorganic nitrogen and growth of maize under chromium stress. Chemosphere, 227, 124–132.
Zhu, Y., et al. (2021). Insight into efficient removal of Cr(VI) by magnetite immobilized with Lysinibacillus sp JLT12: Mechanism and performance. Chemosphere, 262, 127901.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51876018 and 52176178), Scientific and Technological Research Program, Chongqing Municipal Education Commission Foundation (KJQN201801117),Innovation research group of universities in Chongqing (CXQT21035) and The Action Plan for High Quality Development of Graduate Education of Chongqing University of Technology (gzltd202204).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Zhong, D., Xu, Y. et al. Removal of Hexavalent Chromium from Simulated Wastewater by Polyethylene Glycol–Modified D201 Resin-Supported Nanoscale Zero-Valent Iron. Water Air Soil Pollut 233, 446 (2022). https://doi.org/10.1007/s11270-022-05920-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05920-2