Abstract
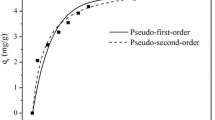
In this study, kenaf, a fast-growing plant, was pyrolyzed to biochar, and the biochar was impregnated with aluminum to improve its fluoride adsorption capacity. The Al-impregnated kenaf biochar (Al-KNF-BC) was pyrolyzed at temperatures of 300–700 °C, where the specimen treated at 300 °C (Al-KNF-300) demonstrated the highest fluoride adsorption capacity. The kinetics and equilibrium adsorption of fluoride by Al-KNF-300 followed the pseudo-second-order and Langmuir models, respectively. According to the Langmuir model, the maximum fluoride adsorption capacity of Al-KNF-300 was 13.93 mg/g. The enthalpy and entropy of fluoride adsorption by Al-KNF-300 were 37.80 kJ/mol and 124.1 J/mol K, respectively. Fluoride adsorption by Al-KNF-300 was favorable at pH values as low as 3, and the effect of anion competition followed the order HCO3− > SO42− > NO3− > Cl−. A maximum adsorption efficiency of 99.23% was obtained at an adsorbent concentration of 16.67 g/L, at which point the fluoride concentration decreased from 100 to < 1.5 mg/L (the drinking water standard). Based on these results, Al-KNF-300 can be considered an effective and inexpensive adsorbent for removing fluoride from contaminated water to meet drinking water standards.






Similar content being viewed by others

Data Availability
All data generated or analyzed during this study are included in the published article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdel-Fattah, T. M., Mahmoud, M. E., Ahmed, S. B., Huff, M. D., Lee, J. W., & Kumar, S. (2015). Biochar from woody biomass for removing metal contaminants and carbon sequestration. Journal of Industrial and Engineering Chemistry, 22, 103–109. https://doi.org/10.1016/j.jiec.2014.06.030
Abdilla, R. M., Rasrendra, C. B., & Heeres, H. J. (2018). Kinetic studies on the conversion of levoglucosan to glucose in water using Brønsted acids as the catalysts. Industrial & Engineering Chemistry Research, 57(9), 3204–3214. https://doi.org/10.1021/acs.iecr.8b00013
Ahamad, K. U., Singh, R., Baruah, I., Choudhury, H., & Sharma, M. R. (2018). Equilibrium and kinetics modeling of fluoride adsorption onto activated alumina, alum and brick powder. Groundwater for Sustainable Development, 7, 452–458. https://doi.org/10.1016/j.gsd.2018.06.005
Ahmed, M. B., Zhou, J. L., Ngo, H. H., Guo, W., & Chen, M. (2016). Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresource Technology, 214, 836–851. https://doi.org/10.1016/j.biortech.2016.05.057
Amini, M., Mueller, K. I. M., Abbaspour, K. C., Rosenberg, T., Afyuni, M., Møller, K. N., Sarr, M., & Johnson, C. A. (2008). Statistical modeling of global geogenic fluoride contamination in groundwaters. Environmental Science & Technology, 42(10), 3662–3668. https://doi.org/10.1021/es071958y
Amor, Z., Bariou, B., Mameri, N., Taky, M., Nicolas, S., & Elmidaoui, A. (2001). Fluoride removal from brackish water by electrodialysis. Desalination, 133(3), 215–223. https://doi.org/10.1016/S0011-9164(01)00102-3
Azwa, Z. N., & Yousif, B. F. (2013). Characteristics of kenaf fibre/epoxy composites subjected to thermal degradation. Polymer Degradation and Stability, 98(12), 2752–2759. https://doi.org/10.1016/j.polymdegradstab.2013.10.008
Babaeivelni, K., & Khodadoust, A. P. (2013). Adsorption of fluoride onto crystalline titanium dioxide: Effect of pH, ionic strength, and co-existing ions. Journal of Colloid and Interface Science, 394, 419–427. https://doi.org/10.1016/j.jcis.2012.11.063
Biswas, K., Bandhoyapadhyay, D., & Ghosh, U. C. (2007). Adsorption kinetics of fluoride on iron (III)-zirconium (IV) hybrid oxide. Adsorption, 13(1), 83–94. https://doi.org/10.1007/s10450-007-9000-1
Biswas, K., Gupta, K., & Ghosh, U. C. (2009). Adsorption of fluoride by hydrous iron (III)–tin (IV) bimetal mixed oxide from the aqueous solutions. Chemical Engineering Journal, 149(1–3), 196–206. https://doi.org/10.1016/j.cej.2008.09.047
Borna, M. O., Pirsaheb, M., Niri, M. V., Mashizie, R. K., Kakavandi, B., Zare, M. R., & Asadi, A. (2016). Batch and column studies for the adsorption of chromium (VI) on low-cost Hibiscus Cannabinus kenaf, a green adsorbent. Journal of the Taiwan Institute of Chemical Engineers, 68, 80–89. https://doi.org/10.1016/j.jtice.2016.09.022
Cengeloglu, Y., Tor, A., Ersoz, M., & Arslan, G. (2006). Removal of nitrate from aqueous solution by using red mud. Separation and Purification Technology, 51(3), 374–378. https://doi.org/10.1016/j.seppur.2006.02.020
Cengloglu, Y., Kir, E., & Ersoz, M. (2002). Removal of fluoride from aqueous solution by using red mud. Journal Separation and Purifaction Technology, 28, 81–86. https://doi.org/10.1016/S1383-5866(02)00016-3
Chaari, I., Fakhfakh, E., Medhioub, M., & Jamoussi, F. (2019). Comparative study on adsorption of cationic and anionic dyes by smectite rich natural clays. Journal of Molecular Structure, 1179, 672–677. https://doi.org/10.1016/j.molstruc.2018.11.039
Chang, M. F., & Liu, J. C. (2007). Precipitation removal of fluoride from semiconductor wastewater. Journal of Environmental Engineering, 133(4), 419–425. https://doi.org/10.1061/(ASCE)0733-9372(2007)133:4(419)
Chen, N., Zhang, Z., Feng, C., Sugiura, N., Li, M., & Chen, R. (2010). Fluoride removal from water by granular ceramic adsorption. Journal of Colloid and Interface Science, 348(2), 579–584. https://doi.org/10.1016/j.jcis.2010.04.048
Chen, N., Zhang, Z., Feng, C., Li, M., Chen, R., & Sugiura, N. (2011). Investigations on the batch and fixed-bed column performance of fluoride adsorption by Kanuma mud. Desalination, 268(1–3), 76–82. https://doi.org/10.1016/j.desal.2010.09.053
Chen, G. J., Peng, C. Y., Fang, J. Y., Dong, Y. Y., Zhu, X. H., & Cai, H. M. (2016). Biosorption of fluoride from drinking water using spent mushroom compost biochar coated with aluminum hydroxide. Desalination and Water Treatment, 57(26), 12385–12395. https://doi.org/10.1080/19443994.2015.1049959
Chen, J., Yang, R., Zhang, Z., & Wu, D. (2022). Removal of fluoride from water using aluminum hydroxide-loaded zeolite synthesized from coal fly ash. Journal of Hazardous Materials, 421, 126817. https://doi.org/10.1016/j.jhazmat.2021.126817
Cho, E. J., Kang, J. K., Moon, J. K., Um, B. H., Lee, C. G., Jeong, S., & Park, S. J. (2021). Removal of triclosan from aqueous solution via adsorption by kenaf-derived biochar: Its adsorption mechanism study via spectroscopic and experimental approaches. Journal of Environmental Chemical Engineering, 9(6), 106343. https://doi.org/10.1016/j.jece.2021.106343
Choi, M. Y., Lee, J. I., Lee, C. G., & Park, S. J. (2021). Feasibility of using calcined Patinopecten yessoensis shells for fluoride removal and investigation of the fluoride removal mechanism. Desalination and Water Treatment, 233, 292–302. https://doi.org/10.5004/dwt.2021.27551
Das, N., Pattanaik, P., & Das, R. (2005). Defluoridation of drinking water using activated titanium rich bauxite. Journal of Colloid and Interface Science, 292(1), 1–10. https://doi.org/10.1016/j.jcis.2005.06.045
Dhawane, S. H., Khan, A. A., Singh, K., Tripathi, A., Hasda, R., & Halder, G. (2018). Insight into optimization, isotherm, kinetics, and thermodynamics of fluoride adsorption onto activated alumina. Environmental Progress & Sustainable Energy, 37(2), 766–776. https://doi.org/10.1002/ep.12814
Dhillon, A., Soni, S. K., & Kumar, D. (2017). Enhanced fluoride removal performance by Ce–Zn binary metal oxide: Adsorption characteristics and mechanism. Journal of Fluorine Chemistry, 199, 67–76. https://doi.org/10.1016/j.jfluchem.2017.05.002
Ding, Z., Xu, X., Phan, T., Hu, X., & Nie, G. (2018). High adsorption performance for As (III) and As (V) onto novel aluminum-enriched biochar derived from abandoned Tetra Paks. Chemosphere, 208, 800–807. https://doi.org/10.1016/j.chemosphere.2018.06.050
do Nascimento, D. C., da Silva, M. G. C., & Vieira, M. G. A. (2021). Adsorption of propranolol hydrochloride from aqueous solutions onto thermally treated bentonite clay: A complete batch system evaluation. Journal of Molecular Liquids, 337, 116442. https://doi.org/10.1016/j.molliq.2021.116442
Ebadi, M., Buskaran, K., Bullo, S., Hussein, M. Z., Fakurazi, S., & Pastorin, G. (2021). Drug delivery system based on magnetic iron oxide nanoparticles coated with (polyvinyl alcohol-zinc/aluminium-layered double hydroxide-sorafenib). Alexandria Engineering Journal, 60(1), 733–747. https://doi.org/10.1016/j.aej.2020.09.061
Elazhar, F., Tahaikt, M., Achatei, A., Elmidaoui, F., Taky, M., El Hannouni, F., Laaziz, I., Jariri, S., Amarari, M. E., & Elmidaoui, A. (2009). Economical evaluation of the fluoride removal by nanofiltration. Desalination, 249(1), 154–157. https://doi.org/10.1016/j.desal.2009.06.017
Fan, X., Parker, D. J., & Smith, M. D. (2003). Adsorption kinetics of fluoride on low cost materials. Water Research, 37(20), 4929–4937. https://doi.org/10.1016/j.watres.2003.08.014
Fan, C., Yin, N., Cai, X., Du, X., Wang, P., Liu, X., Li, Y., Chang, X., Du, H., Ma, J., & Cui, Y. (2022). Stabilization of fluorine-contaminated soil in aluminum smelting site with biochar loaded iron-lanthanide and aluminum-lanthanide bimetallic materials. Journal of Hazardous Materials, 426, 128072. https://doi.org/10.1016/j.jhazmat.2021.128072
Farooqi, A., Masuda, H., & Firdous, N. (2007). Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environmental Pollution, 145(3), 839–849. https://doi.org/10.1016/j.envpol.2006.05.007
Farrah, H., Slavek, J., & Pickering, W. F. (1987). Fluoride interactions with hydrous aluminum oxides and alumina. Soil Research, 25(1), 55–69. https://doi.org/10.1071/SR9870055
Feng, N., Guo, X., Liang, S., Zhu, Y., & Liu, J. (2011). Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. Journal of Hazardous Materials, 185(1), 49–54. https://doi.org/10.1016/j.jhazmat.2010.08.114
Ferrara, F., Orsini, A., Plaisant, A., & Pettinau, A. (2014). Pyrolysis of coal, biomass and their blends: Performance assessment by thermogravimetric analysis. Bioresource Technology, 171, 433–441. https://doi.org/10.1016/j.biortech.2014.08.104
Freundlich, H. (1907). Über die adsorption in lösungen. Zeitschrift Für Physikalische Chemie, 57(1), 385–470. https://doi.org/10.1515/zpch-1907-5723
Ghorai, S., & Pant, K. K. (2004). Investigations on the column performance of fluoride adsorption by activated alumina in a fixed-bed. Chemical Engineering Journal, 98(1–2), 165–173. https://doi.org/10.1016/j.cej.2003.07.003
Guo, Y., & Rockstraw, D. A. (2006). Physical and chemical properties of carbons synthesized from xylan, cellulose, and Kraft lignin by H3PO4 activation. Carbon, 44(8), 1464–1475. https://doi.org/10.1016/j.carbon.2005.12.002
Gupta, V. K., Ali, I., & Saini, V. K. (2007). Defluoridation of wastewaters using waste carbon slurry. Water Research, 41(15), 3307–3316. https://doi.org/10.1016/j.watres.2007.04.029
Ha, E. S., Ha, D. H., Kuk, D. H., Sim, W. Y., Baek, I. H., Kim, J. S., Park, H. J., & Kim, M. S. (2017). Solubility of cilostazol in the presence of polyethylene glycol 4000, polyethylene glycol 6000, polyvinylpyrrolidone K30, and poly (1-vinylpyrrolidone-co-vinyl acetate) at different temperatures. The Journal of Chemical Thermodynamics, 113, 6–10. https://doi.org/10.1016/j.jct.2017.05.040
Harrington, L. F., Cooper, E. M., & Vasudevan, D. (2003). Fluoride sorption and associated aluminum release in variable charge soils. Journal of Colloid and Interface Science, 267(2), 302–313. https://doi.org/10.1016/S0021-9797(03)00609-X
Hasfalina, C. M., Maryam, R. Z., Luqman, C. A., & Rashid, M. (2010). The potential use of kenaf as a bioadsorbent for the removal of copper and nickel from single and binary aqueous solution. Journal of Natural Fibers, 7(4), 267–275. https://doi.org/10.1080/15440478.2010.527508
Hasfalina, C. M., Maryam, R. Z., Luqman, C. A., & Rashid, M. (2012). Adsorption of copper (II) from aqueous medium in fixed-bed column by kenaf fibres. Asia-Pacific Chemical, Biological & Environmental Engineering Society, 3, 255–263. https://doi.org/10.1016/j.apcbee.2012.06.079
He, Y., Zhang, L., An, X., Wan, G., Zhu, W., & Luo, Y. (2019). Enhanced fluoride removal from water by rare earth (La and Ce) modified alumina: Adsorption isotherms, kinetics, thermodynamics and mechanism. Science of the Total Environment, 688, 184–198. https://doi.org/10.1016/j.scitotenv.2019.06.175
Ho, Y. S., & McKay, G. (1998). Kinetic model for lead (II) sorption on to peat. Adsorption Science & Technology, 16(4), 243–255. https://doi.org/10.1177/026361749801600401
Iriel, A., Bruneel, S. P., Schenone, N., & Cirelli, A. F. (2018). The removal of fluoride from aqueous solution by a lateritic soil adsorption: Kinetic and equilibrium studies. Ecotoxicology and Environmental Safety, 149, 166–172. https://doi.org/10.1016/j.ecoenv.2017.11.016
Isik, Z., Saleh, M., M’barek, I., Yabalak, E., Dizge, N., & Deepanraj, B. (2022). Investigation of the adsorption performance of cationic and anionic dyes using hydrochared waste human hair. Biomass Conversion and Biorefinery, in press. https://doi.org/10.1007/s13399-022-02582-2
Islam, M., & Patel, R. K. (2007). Evaluation of removal efficiency of fluoride from aqueous solution using quick lime. Journal of Hazardous Materials, 143(1–2), 303–310. https://doi.org/10.1016/j.jhazmat.2006.09.030
Jagtap, S., Yenkie, M. K. N., Labhsetwar, N., & Rayalu, S. (2011). Defluoridation of drinking water using chitosan based mesoporous alumina. Microporous and Mesoporous Materials, 142(2–3), 454–463. https://doi.org/10.1016/j.micromeso.2010.12.028
Jones, S., Burt, B. A., Petersen, P. E., & Lennon, M. A. (2005). The effective use of fluorides in public health. Bulletin of the World Health Organization, 83, 670–676. https://doi.org/10.1590/S0042-96862005000900012
Karthikeyan, G., Pius, A., & Alagumuthu, G. (2005). Fluoride adsorption studies of montmorillonite clay. Indian Journal of Chemical Technology, 12:263–272. http://nopr.niscair.res.in/handle/123456789/8640
Kasperiski, F. M., Lima, E. C., Umpierres, C. S., dos Reis, G. S., Thue, P. S., Lima, D. R., Dias, S. L. P., Saucier, C., & da Costa, J. B. (2018). Production of porous activated carbons from Caesalpinia ferrea seed pod wastes: Highly efficient removal of captopril from aqueous solutions. Journal of Cleaner Production, 197, 919–929. https://doi.org/10.1016/j.jclepro.2018.06.146
Khan, T. A., & Khan, E. A. (2015). Removal of basic dyes from aqueous solution by adsorption onto binary iron-manganese oxide coated kaolinite: Non-linear isotherm and kinetics modeling. Applied Clay Science, 107, 70–77. https://doi.org/10.1016/j.clay.2015.01.005
Kim, S. J., & Um, B. H. (2020). Process variable optimization of kenaf two-stage fractionation based on dilute hydrochloric acid followed by ethanol organosolv for component separation. BioEnergy Research, 13(1), 249–259. https://doi.org/10.1007/s12155-019-10057-y
Kusrini, E., Sofyan, N., Suwartha, N., Yesya, G., & Priadi, C. R. (2015). Chitosan-praseodymium complex for adsorption of fluoride ions from water. Journal of Rare Earths, 33(10), 1104–1113. https://doi.org/10.1016/S1002-0721(14)60533-0
Lagergren, S. K. (1898). About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl, 24, 1–39.
Langmuir, I. (1916). The constitution and fundamental properties of solids and liquids. Part I. Solids. Journal of the American chemical society, 38(11), 2221–2295. https://doi.org/10.1021/ja02268a002
Lee, G., Chen, C., Yang, S. T., & Ahn, W. S. (2010). Enhanced adsorptive removal of fluoride using mesoporous alumina. Microporous and Mesoporous Materials, 127(1–2), 152–156. https://doi.org/10.1016/j.micromeso.2009.07.007
Lee, J. I., Hong, S. H., Lee, C. G., & Park, S. J. (2021). Fluoride removal by thermally treated egg shells with high adsorption capacity, low cost, and easy acquisition. Environmental Science and Pollution Research, 28(27), 35887–35901. https://doi.org/10.1007/s11356-021-13284-z
Lee, J. I., Kang, J. K., Hong, S. H., Lee, C. G., Jeong, S., & Park, S. J. (2021b). Thermally treated Mytilus coruscus shells for fluoride removal and their adsorption mechanism. Chemosphere, 263, 128328. https://doi.org/10.1016/j.chemosphere.2020.128328
Leyva-Ramos, R., Rivera-Utrilla, J., Medellin-Castillo, N. A., & Sanchez-Polo, M. (2010). Kinetic modeling of fluoride adsorption from aqueous solution onto bone char. Chemical Engineering Journal, 158(3), 458–467. https://doi.org/10.1016/j.cej.2010.01.019
Li, Y. H., Wang, S., Cao, A., Zhao, D., Zhang, X., Xu, C., Luan, Z., Ruan, D., Liang, J., Wu, D., & Wei, B. (2001). Adsorption of fluoride from water by amorphous alumina supported on carbon nanotubes. Chemical Physics Letters, 350(5–6), 412–416. https://doi.org/10.1016/S0009-2614(01)01351-3
Lin, B., Zhou, J., Qin, Q., Song, X., & Luo, Z. (2019). Thermal behavior and gas evolution characteristics during co-pyrolysis of lignocellulosic biomass and coal: A TG-FTIR investigation. Journal of Analytical and Applied Pyrolysis, 144, 104718. https://doi.org/10.1016/j.jaap.2019.104718
Mahfoudhi, N., & Boufi, S. (2020). Porous material from cellulose nanofibrils coated with aluminum hydroxyde as an effective adsorbent for fluoride. Journal of Environmental Chemical Engineering, 8(3), 103779. https://doi.org/10.1016/j.jece.2020.103779
Mahmoud, D. K., Salleh, M. A. M., Karim, W. A. W. A., Idris, A., & Abidin, Z. Z. (2012). Batch adsorption of basic dye using acid treated kenaf fibre char: Equilibrium, kinetic and thermodynamic studies. Chemical Engineering Journal, 181, 449–457. https://doi.org/10.1016/j.cej.2011.11.116
Malik, D. S., Jain, C. K., & Yadav, A. K. (2017). Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Applied Water Science, 7(5), 2113–2136. https://doi.org/10.1007/s13201-016-0401-8
Mayakaduwa, S. S., Kumarathilaka, P., Herath, I., Ahmad, M., Al-Wabel, M., Ok, Y. S., Usman, A., Abduljabbar, A., & Vithanage, M. (2016). Equilibrium and kinetic mechanisms of woody biochar on aqueous glyphosate removal. Chemosphere, 144, 2516–2521. https://doi.org/10.1016/j.chemosphere.2015.07.080
Medellin-Castillo, N. A., Leyva-Ramos, R., Ocampo-Perez, R., Garcia de la Cruz, R. F., Aragon-Pina, A., Martinez-Rosales, J. M., Guerrero-Coronado, R. M., & Fuentes-Rubio, L. (2007). Adsorption of fluoride from water solution on bone char. Industrial & Engineering Chemistry Research, 46(26), 9205–9212. https://doi.org/10.1021/ie070023n
Meilani, V., Lee, J. I., Kang, J. K., Lee, C. G., Jeong, S., & Park, S. J. (2021). Application of aluminum-modified food waste biochar as adsorbent of fluoride in aqueous solutions and optimization of production using response surface methodology. Microporous and Mesoporous Materials, 312, 110764. https://doi.org/10.1016/j.micromeso.2020.110764
Mian, M. M., & Liu, G. (2018). Recent progress in biochar-supported photocatalysts: Synthesis, role of biochar, and applications. Rsc Advances, 8(26), 14237–14248. https://doi.org/10.1039/C8RA02258E
Mirsoleimani-azizi, S. M., Setoodeh, P., Zeinali, S., & Rahimpour, M. R. (2018). Tetracycline antibiotic removal from aqueous solutions by MOF-5: Adsorption isotherm, kinetic and thermodynamic studies. Journal of Environmental Chemical Engineering, 6(5), 6118–6130. https://doi.org/10.1016/j.jece.2018.09.017
Mohan, D., Kumar, S., & Srivastava, A. (2014). Fluoride removal from ground water using magnetic and nonmagnetic corn stover biochars. Ecological Engineering, 73, 798–808. https://doi.org/10.1016/j.ecoleng.2014.08.017
Nishino, T., Hirao, K., Kotera, M., Nakamae, K., & Inagaki, H. (2003). Kenaf reinforced biodegradable composite. Composites Science and Technology, 63(9), 1281–1286. https://doi.org/10.1016/S0266-3538(03)00099-X
Onyango, M. S., Kojima, Y., Aoyi, O., Bernardo, E. C., & Matsuda, H. (2004). Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation-exchanged zeolite F-9. Journal of Colloid and Interface Science, 279(2), 341–350. https://doi.org/10.1016/j.jcis.2004.06.038
Podgorny, P. C., & McLaren, L. (2015). Public perceptions and scientific evidence for perceived harms/risks of community water fluoridation: An examination of online comments pertaining to fluoridation cessation in Calgary in 2011. Canadian Journal of Public Health, 106(6), e413–e425. https://doi.org/10.17269/CJPH.106.5031
Rahman, M. L., Biswas, T. K., Sarkar, S. M., Yusoff, M. M., Sarjadi, M. S., Arshad, S. E., & Musta, B. (2017). Adsorption of rare earth metals from water using a kenaf cellulose-based poly (hydroxamic acid) ligand. Journal of Molecular Liquids, 243, 616–623. https://doi.org/10.1016/j.molliq.2017.08.096
Ramos-Vargas, S., Alfaro-Cuevas-Villanueva, R., Huirache-Acuña, R., & Cortés-Martínez, R. (2018). Removal of fluoride and arsenate from aqueous solutions by aluminum-modified guava seeds. Applied Sciences, 8(10), 1807. https://doi.org/10.3390/app8101807
Rapacz-Kmita, A., Paluszkiewicz, C., Ślósarczyk, A., & Paszkiewicz, Z. (2005). FTIR and XRD investigations on the thermal stability of hydroxyapatite during hot pressing and pressureless sintering processes. Journal of Molecular Structure, 744, 653–656. https://doi.org/10.1016/j.molstruc.2004.11.070
Rojas-Mayorga, C. K., Bonilla-Petriciolet, A., Silvestre-Albero, J., Aguayo-Villarreal, I. A., & Mendoza-Castillo, D. I. (2015). Physico-chemical characterization of metal-doped bone chars and their adsorption behavior for water defluoridation. Applied Surface Science, 355, 748–760. https://doi.org/10.1016/j.apsusc.2015.07.163
Saleh, M., Isik, Z., Yabalak, E., Yalvac, M., & Dizge, N. (2021). Green production of hydrochar nut group from waste materials in subcritical water medium and investigation of their adsorption performance for Crystal Violet. Water Environment Research, 93(12), 3075–3089. https://doi.org/10.1002/wer.1659
Salifu, A., Petrusevski, B., Ghebremichael, K., Modestus, L., Buamah, R., Aubry, C., & Amy, G. L. (2013). Aluminum (hydr) oxide coated pumice for fluoride removal from drinking water: Synthesis, equilibrium, kinetics and mechanism. Chemical Engineering Journal, 228, 63–74. https://doi.org/10.1016/j.cej.2013.04.075
Samadi, M. T., Zarrabi, M., Sepehr, M. N., Ramhormozi, S. M., Azizian, S., & Amrane, A. (2014). Removal of fluoride ions by ion exchange resin: kinetic and equilibrium studies. Environmental Engineering & Management Journal,13(1). http://omicron.ch.tuiasi.ro/EEMJ/
Sellers, T., & Reichert, N. A. (1999). Kenaf properties, processing and products. Mississippi State University.
Shahmoradi, A. R., Talebibahmanbigloo, N., Javidparvar, A. A., Bahlakeh, G., & Ramezanzadeh, B. (2020). Studying the adsorption/inhibition impact of the cellulose and lignin compounds extracted from agricultural waste on the mild steel corrosion in HCl solution. Journal of Molecular Liquids, 304, 112751. https://doi.org/10.1016/j.molliq.2020.112751
Shamsuddin, M. S., Yusoff, N. R. N., & Sulaiman, M. A. (2016). Synthesis and characterization of activated carbon produced from kenaf core fiber using H3PO4 activation. Procedia Chemistry, 19, 558–565. https://doi.org/10.1016/j.proche.2016.03.053
Stefaniuk, M., & Oleszczuk, P. (2015). Characterization of biochars produced from residues from biogas production. Journal of Analytical and Applied Pyrolysis, 115, 157–165. https://doi.org/10.1016/j.jaap.2015.07.011
Sun, Z. X., Zheng, T. T., Bo, Q. B., Du, M., & Forsling, W. (2008). Effects of calcination temperature on the pore size and wall crystalline structure of mesoporous alumina. Journal of Colloid and Interface Science, 319(1), 247–251. https://doi.org/10.1016/j.jcis.2007.11.023
Sun, Y., Fang, Q., Dong, J., Cheng, X., & Xu, J. (2011). Removal of fluoride from drinking water by natural stilbite zeolite modified with Fe (III). Desalination, 277(1–3), 121–127. https://doi.org/10.1016/j.desal.2011.04.013
Tang, Y., Guan, X., Su, T., Gao, N., & Wang, J. (2009). Fluoride adsorption onto activated alumina: Modeling the effects of pH and some competing ions. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 337(1–3), 33–38. https://doi.org/10.1016/j.colsurfa.2008.11.027
Tang, Y., Guan, X., Su, T., Gao, N., & Wang, J. (2009). Fluoride adsorption onto activated alumina: Modeling the effects of pH and some competing ions. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 337(1–3), 33–38. https://doi.org/10.1016/j.jhazmat.2009.06.079
Tchomgui-Kamga, E., Alonzo, V., Nanseu-Njiki, C. P., Audebrand, N., Ngameni, E., & Darchen, A. (2010). Preparation and characterization of charcoals that contain dispersed aluminum oxide as adsorbents for removal of fluoride from drinking water. Carbon, 48(2), 333–343. https://doi.org/10.1016/j.carbon.2009.09.034
Thue, P. S., Adebayo, M. A., Lima, E. C., Sieliechi, J. M., Machado, F. M., Dotto, G. L., Vaghetti, J. C. P., & Dias, S. L. (2016). Preparation, characterization and application of microwave-assisted activated carbons from wood chips for removal of phenol from aqueous solution. Journal of Molecular Liquids, 223, 1067–1080. https://doi.org/10.1016/j.molliq.2016.09.032
Tripathy, S. S., Bersillon, J. L., & Gopal, K. (2006). Removal of fluoride from drinking water by adsorption onto alum-impregnated activated alumina. Separation and Purification Technology, 50(3), 310–317. https://doi.org/10.1016/j.seppur.2005.11.036
Turner, B. D., Binning, P., & Stipp, S. L. S. (2005). Fluoride removal by calcite: Evidence for fluorite precipitation and surface adsorption. Environmental Science & Technology, 39(24), 9561–9568. https://doi.org/10.1021/es0505090
United States Environmental Protection Agency. (2010, July). Basic information about fluoride in drinking water. Retrieved May 2022, from https://tdb.epa.gov/tdb/contaminant?id=10700
Wang, Y., & Reardon, E. J. (2001). Activation and regeneration of a soil sorbent for defluoridation of drinking water. Applied Geochemistry, 16(5), 531–539. https://doi.org/10.1016/S0883-2927(00)00050-0
Wang, S. G., Ma, Y., Shi, Y. J., & Gong, W. X. (2009). Defluoridation performance and mechanism of nano-scale aluminum oxide hydroxide in aqueous solution Journal of Chemical Technology & Biotechnology: International Research in Process. Environmental & Clean Technology, 84(7), 1043–1050. https://doi.org/10.1002/jctb.2131
Wang, G., Zhang, S., Yao, P., Chen, Y., Xu, X., Li, T., & Gong, G. (2018). Removal of Pb (II) from aqueous solutions by Phytolacca americana L biomass as a low cost biosorbent. Arabian Journal of Chemistry, 11(1), 99–110. https://doi.org/10.1016/j.arabjc.2015.06.011
World Health Organization (WHO). (2017). Guidelines for drinking—Water quality, 4th edition, incorporating the 1st addendum.
Yusof, S. R. M., Zahri, N. A. M., Koay, Y. S., Nourouzi, M. M., Chuah, L. A., & Choong, T. S. Y. (2015). Removal of fluoride using modified kenaf as adsorbent. Journal of Engineering Science and Technology, 10(11).
Zhang, G., Zhang, Q., Sun, K., Liu, X., Zheng, W., & Zhao, Y. (2011). Sorption of simazine to corn straw biochars prepared at different pyrolytic temperatures. Environmental Pollution, 159(10), 2594–2601. https://doi.org/10.1016/j.envpol.2011.06.012
Zhang, Y., Lin, X., Zhou, Q., & Luo, X. (2016). Fluoride adsorption from aqueous solution by magnetic core-shell Fe3O4@ alginate-La particles fabricated via electro-coextrusion. Applied Surface Science, 389, 34–45. https://doi.org/10.1016/j.envpol.2011.06.012
Zhang, H., Ruan, Y., Feng, Y., Su, M., Diao, Z., Chen, D., Hou, L., Lee, P. H., & Kong, L. (2019). Solvent-free hydrothermal synthesis of gamma-aluminum oxide nanoparticles with selective adsorption of Congo red. Journal of Colloid and Interface Science, 536, 180–188. https://doi.org/10.1016/j.jcis.2018.10.054
Zhou, N., Guo, X., Ye, C., Yan, L., Gu, W., Wu, X., Zhou, Q., & Cheng, Q. (2022). Enhanced fluoride removal from drinking water in wide pH range using La/Fe/Al oxides loaded rice straw biochar. Water Supply, 22(1), 779–794. https://doi.org/10.2166/ws.2021.232
Zhuang, Z., Wang, L., & Tang, J. (2021). Efficient removal of volatile organic compound by ball-milled biochars from different preparing conditions. Journal of Hazardous Materials, 406, 124676. https://doi.org/10.1016/j.jhazmat.2020.124676
Funding
This work was supported by the Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01477903) of the Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Contributions
MYC: writing—original draft, experimental investigation, data analysis; CGL: writing—review and editing; SJP: conceptualization, writing—review and editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Consent for Participation
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, MY., Lee, CG. & Park, SJ. Enhanced Fluoride Adsorption on Aluminum-Impregnated Kenaf Biochar: Adsorption Characteristics and Mechanism. Water Air Soil Pollut 233, 435 (2022). https://doi.org/10.1007/s11270-022-05906-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05906-0