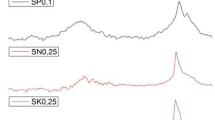
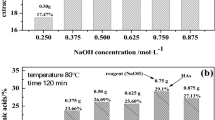
Abstract
The goal of this research is to evaluate waste lignocellulosic materials for the production humic acid (HA) as a natural, low-cost, and effective adsorbent for heavy metals (Ni, Cr, and Cu) removal from aqueous medium. The fourier transform infrared (FTIR) spectroscopy, fluoresence spectroscopy, LC-ESI-TOF/MS, and stable carbon isotopes analysis were applied to compare the chemical structure of humic acids obtained from wheat straw (C3) and corn straw (C4). The adsorption efficiency of humic acids obtained from different lignocellulosic sources was investigated and compared to the commercial HA. The adsorption efficiency for Cu ions was almost 70% and the metal adsorption capacity of corn-HA is remarkably higher than commercial HA. Ni ions exhibited the lowest adsorption percentage for which the removal reached 21.0% with corn-HA. This work showed that lignocellulosic-derived humic acids are suitable adsorbent for heavy metals and can be used for cleaning of waters or other systems with low concentrations of metal ions.





Similar content being viewed by others
Data Availability
Research data are not shared.
References
Ahmed, O. H., Husni, M. H. A., Anuar, A. R., Hanafi, M. M., & Angela, E. D. S. (2005). A modified way of producing humic acid from composted pineapple leaves. Journal of Sustainable Agriculture and Environment, 25(1), 129–139.
Aoyama, M. (2001). Do humic substances exhibit fluorescence? Understanding and managing organic matter in soils, sediments and waters. Proceeding of the 9 th International Conference of the International Humic Substances Society University of Adelaide, Adelaide, Australia, 21 st– 25st September 1998. Editors R.S. Swift and K.M. Spark.
ASTM Standart D–1102–84 (2013) Standard test method for ash in wood. Retrieved December 15, 2021, from www.astm.org.
ASTM Standart D-2016-74 (1983). Methods of test for moisture content of wood. Retrieved November, 2021, from www.astm.org.
Başçetinçelik, A., Öztürk, H. H., Karaca, C., Kaçira, M., Ekinci, K., Kaya, D., Baban, A., Güneş, K., Komitti, N., Barnes, I., & Nieminen, M. (2005). Türkiye’de Tarimsal Atiklarin Değerlendirilmesi Rehberi, LIFE 03 TCY/ TR /000061, 2005. Adana.
Ceh, H., & Hadzi, D. (1956). Infrared spectra of humic acids and their derivatives. Fuel, 55, 77–83.
Čežíková, J., Kozler, J., Madronová, L., Novák, J., & Janoš, P. (2001). Humic acids from coals of the North-Bohemian coal field-II. Metal-binding capacity under static conditions, Reactive & Functional Polymers, 47(2), 111–118.
Duncan, D. A., Bodle, W. W., Banejerd, D. P. (1981). Energy from biomass and waste. 5th Symposium, Papers: Institute of Gas Technology, Chicago, pp-917.
Farmer, C., & Moreison, R. I. (1960). Chemical and infrared studies on phragmites peat and its humio acid. PPOC. Royal D&&n Sot. A, 1, 85–104.
Ferro-García, M. A., Rivera-Utrilla, J., Bautista-Toledo, I., & Moreno-Castilla, C. (1998). Adsorption of humic substances on activated carbon from aqueous solutions and their effect on the removal of Cr(III) ions. Langmuir, 14(7), 1880–1886.
Foyle, T., Jennings, L., & Mulcahy, P. (2007). Compositional analysis of lignocellulosic materials: Evaluation of methods used for sugar analysis of waste paper and straw. Bioresource Technology, 98, 3026–3036.
González Pérez, M., Martin-Neto, L., Saab, S. C., Novotny, E. H., Milori Débora, M. B. P., Bagnato, V. S., Colnago, L. A., Melo, W. J., & Knicker, H. (2004). Characterization of humic acids from a Brazilian Oxisol under different tillage systems by EPR, 13C NMR, FTIR and fluorescence spectroscopy. Geoderma, 118, 181–190.
Havelcová, M., Mizera, J., Śykorová, I., & Pekař, M. (2009). Sorption of metal ions on lignite and the derived humic substances. Journal of Hazardous Materials, 161(1), 559–564.
Hiradate, S., Nakadai, T., Shindo, H., & Yoneyama, T. (2004). Carbon source of humic substances in some Japanese volcanic ash soils determined by carbon stable isotopic ratio, δ13C. Geoderma, 119(1–2), 133–141.
Kanduč, T., Markič, M., & Pezdič, J. (2005). Stable isotope geochemistry of different lithotypes of the Velenje lignite (Slovenia). Geologija, 48(1), 83–95.
Kiprop, K. J., Coumon, M. C., Pourtier, E., Kimutai, S., & Kirui, S. (2013). Synthesis of humic and fulvic acids and their characterization using optical spectroscopy (ATR-FTIR and UV–visible). International Journal of Applied Science and Technology, 3(8), 28–35.
Kislenko, V. N., & Oliinyk, L. P. (2003). Binding of copper(II), cobalt(II), and nickel(II) cations with humic acids and their sodium salts in aqueous media. Russian Journal of Applied Chemistry, 76(12), 1962–1964.
Klöcking, R. (1992). Humic substances in the global environment and implications in human health. N. Senesiand T. M. Miano (Eds.), Proc. 6th Intern. Meeting of the Intern. Humic Substances Soc., Monopoli, Bari, Italy, September 20-25, p.129, Elsevier.
Klučáková, M., & Pekař, M. (2006). New model for equilibrium sorption of metal ions on solid humic acids. Colloids and Surfaces A: Physicochemical and Engineering, 286(13), 126–133.
Maccarthy, P. (2001). The principles of humic substances: an introduction to the first principle. Soil Science, 166, 738–751.
Meryemoglu, B., Hesenov, A., Irmak, S., Atanur, O. M., & Erbatur, O. (2010). Aqueous phase reforming of biomass using various types of supported precious metal and raney nickel catalysts for hydrogen production. International Journal of Hydrogen Energy, 35(22), 12580–12587.
Miano, T. M. and Alberts, J. (2001). Fluorescence behavior of molecular fractions of suwannee river water. The effect of photo-oxidation. In Understanding Humic Substances. Advanced Methods, Properties and Applications. Ed. E. A. Ghabbor and G. Davies. Boston. USA 157 -166.
Milori, D. M. B. P. L., Bayer, M. N. C., Mielniczuk, J., & Bagnato, V. S. (2002). Humification degree of soil humic acids determined by fluorescence spectroscopy. Soil Science, 167(11), 739–749.
Motta, F. L., & Santana, M. H. A. (2013). Production of humic acids from oil palm empty fruit bunch by submerged fermentation with Trichoderma viride: cellulosic substrates and nitrogen sources. Biotechnology Progress, 29, 631–637.
Padovan, G. J., Rodriques, L. P., Leme, I. A., Jong, D. D., & Marchini, J. S. (2007). Presence of C4 sugars in honey samples detected by the carbon isotope ratio measured by IRMS. Eurasian Journal of Analytical Chemistry, 2(3), 134–141.
Palanivell, P., Susilawati, K., Ahmed, O. H., & Majid, N. K. (2013). Compost and crude humic substances produced from selected wastes and their effects on Zea mays L. nutrient uptake and growth. The Scientific World Journal, 2013, 1-15.
Schnitzer, M. (1965). The application of infrared spectroscopy to investigations on soil humio compounds. Can. Spectroae, 10, 121–127.
Schulten, H. R., & Schnitzer, M. (1997). The chemistry of soil organic nitrogen: a review. Soil Science, 162, 115.
Sławińska, D., Rolewski, P., & Sławiński, J. (2007). Synthesis and properties of model humic substances derived from gallic acid. International Agrophysics, 21(2), 199–208.
Sun, R. C., & Tomkinson, J. (2002). Characterizations of hemicelluloses obtained by classical and ultrasonically assisted extractions from wheat straw. Carbohydrate Polymers, 50, 263–271.
Theng, B. K. G., & Posner, A. M. (1967). Nature of the carbonyl groups in soil humio acid. Soil Science, 104, 191–201.
Verstraete, W., & Devliegher, W. (1997). Formation of non-bioavailable organic residues in soil: perspectives for site remediation. Biodegradation, 7, 471–485.
Wagner, G. H., & Stevenson, J. (1965). Struotural arrangement of functional groups in soil humic acid so revealed by infrared analyses. Soil Science Society of America Journal, 29, 43–48.
Yang, F., Du, Q., Sui, L., & Cheng, K. (2021). One-step fabrication of artificial humic acid-functionalized colloid-like magnetic biochar for rapid heavy metal removal. Bioresource Technology, 328(124), 825.
Yaşar, S. (2002). Comparison of cellulose, hemicellulose and lignin content in Miscanthus (Elephant Grass) Giganteus, Miscanthus Goliath and Miscanthus Silver Vane. Journal of Süleyman Demirel University Faculty of Forestry, 2, 27–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meryemoglu, B., Ozsel, B.K. Humic Acids Derived from Lignocellulosic Biomass: Characterization and Utilizing for Environmental Applications. Water Air Soil Pollut 233, 402 (2022). https://doi.org/10.1007/s11270-022-05866-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05866-5