Abstract
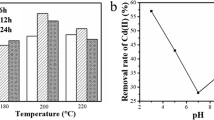
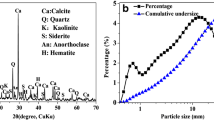
Cd(II) pollution in water will cause serious threats to the environment and human health. The general remediation protocol for Cd(II) pollution by hydroxide (Ca(OH)2 or NaOH) is still faced with filtering difficulties and high effluent pH. To develop an applicable method for the Cd(II) removal in water, we prepared an activated calcium carbonate (CaCO3) material by changing the crystallinity, particle size, and surface activity of CaCO3, and investigated the corresponding Cd(II) removal capacity from aqueous solutions. The results showed that more than 99.9% of Cd(II) was removed within 10 min in an initial concentration of 50 mg L−1. Moreover, the effluent pH is close to neutral after removal of Cd(II), and the sediment is filtered well. Comparative experiments and characterizations have demonstrated that the excellent Cd(II) removal performance of activated CaCO3 is due to the mechanical activation changes the surface activity of the original stable CaCO3, promotes the slow-release dissolution equilibrium of the active carbonate groups, and thus the coprecipitation of cadmium hydroxide and cadmium carbonate on CaCO3 particles. This research demonstrates that mechanical activation of CaCO3 could be used as a repair material for efficient removal of heavy metal pollution in water, which can solve the problems of filtration and high effluent pH of alkaline precipitation.








Similar content being viewed by others
Data Availability
All data are given in the manuscript.
References
Ahmed, M. F., Mokhtar, M. B., Alam, L., Mohamed, C. A. R., & Ta, G. C. (2020). Investigating the status of cadmium, chromium and lead in the drinking water supply chain to ensure drinking water quality in malaysia. Water (switzerland), 12(10), 1–26. https://doi.org/10.3390/w12102653
Anwar, A., & Akbar, S. (2018). Continuous microwave assisted flow synthesis and characterization of calcium deficient hydroxyapatite nanorods. Advanced Powder Technology, 29(6), 1493–1498. https://doi.org/10.1016/j.apt.2018.03.014
Chen, Q., Luo, Z., Hills, C., Xue, G., & Tyrer, M. (2009). Precipitation of heavy metals from wastewater using simulated flue gas: Sequent additions of fly ash, lime and carbon dioxide. Water Research, 43(10), 2605–2614. https://doi.org/10.1016/j.watres.2009.03.007
Chen, B., Bao, S., & Zhang, Y. (2021). Synergetic strengthening mechanism of ultrasound combined with calcium fluoride towards vanadium extraction from low-grade vanadium-bearing shale. International Journal of Mining Science and Technology, 31(6), 1095–1106. https://doi.org/10.1016/j.ijmst.2021.07.008
Chen, B., Bao, S., Zhang, Y., & Ren, L. (2022). A novel and sustainable technique to precipitate vanadium from vanadium-rich solutions via efficient ultrasound irradiation. Journal of Cleaner Production, 339, 130755. https://doi.org/10.1016/j.jclepro.2022.130755
Fu, F., & Wang, Q. (2011). Removal of heavy metal ions from wastewaters: A review. Journal of Environmental Management, 92(3), 407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Heuss-Aßbichler, S., John, M., Klapper, D., Bläß, U. W., & Kochetov, G. (2016). Recovery of copper as zero-valent phase and/or copper oxide nanoparticles from wastewater by ferritization. Journal of Environmental Management, 181, 1–7. https://doi.org/10.1016/j.jenvman.2016.05.053
Hu, H., Li, X., Huang, P., Zhang, Q., & Yuan, W. (2017). Efficient removal of copper from wastewater by using mechanically activated calcium carbonate. Journal of Environmental Management, 203, 1–7. https://doi.org/10.1016/j.jenvman.2017.07.066
Kefeni, K. K., Mamba, B. B., & Msagati, T. A. M. (2017). Magnetite and cobalt ferrite nanoparticles used as seeds for acid mine drainage treatment. Journal of Hazardous Materials, 333, 308–318. https://doi.org/10.1016/j.jhazmat.2017.03.054
Lambert, A., Drogui, P., Daghrir, R., Zaviska, F., & Benzaazoua, M. (2014). Removal of copper in leachate from mining residues using electrochemical technology. Journal of Environmental Management, 133, 78–85. https://doi.org/10.1016/j.jenvman.2013.11.036
Leukel, S., Panthöfer, M., Mondeshki, M., Kieslich, G., Wu, Y., Krautwurst, N., & Tremel, W. (2018). Mechanochemical access to defect-stabilized amorphous calcium carbonate. Chemistry of Materials, 30(17), 6040–6052. https://doi.org/10.1021/acs.chemmater.8b02339
Li, J., & Hitch, M. (2018). Mechanical activation of magnesium silicates for mineral carbonation, a review. Minerals Engineering, 128, 69–83. https://doi.org/10.1016/j.mineng.2018.08.034
Li, X., Lei, Z., Qu, J., Li, Z., Zhou, X., & Zhang, Q. (2017). Synthesizing slow-release fertilizers via mechanochemical processing for potentially recycling the waste ferrous sulfate from titanium dioxide production. Journal of Environmental Management, 186, 120–126. https://doi.org/10.1016/j.jenvman.2016.10.058
Lin, P. Y., Wu, H. M., Hsieh, S. L., Li, J. S., Dong, C., Chen, C. W., & Hsieh, S. (2020). Preparation of vaterite calcium carbonate granules from discarded oyster shells as an adsorbent for heavy metal ions removal. Chemosphere, 254, 126903. https://doi.org/10.1016/j.chemosphere.2020.126903
Lundström, M., Liipo, J., Taskinen, P., & Aromaa, J. (2016). Copper precipitation during leaching of various copper sulfide concentrates with cupric chloride in acidic solutions. Hydrometallurgy, 166, 136–142. https://doi.org/10.1016/j.hydromet.2016.10.017
Panja, S., Hanson, S., & Wang, C. (2020). EDTA-inspired polydentate hydrogels with exceptionally high heavy metal adsorption capacity as reusable adsorbents for wastewater purification. ACS Applied Materials and Interfaces, 12(22), 25276–25285. https://doi.org/10.1021/acsami.0c03689
Ramola, S., Belwal, T., Li, C. J., Wang, Y. Y., Lu, H. H., Yang, S. M., & Zhou, C. H. (2020). Improved lead removal from aqueous solution using novel porous bentonite - and calcite-biochar composite. Science of the Total Environment, 709, 136171. https://doi.org/10.1016/j.scitotenv.2019.136171
Šepelák, V., Düvel, A., Wilkening, M., Becker, K. D., & Heitjans, P. (2013). Mechanochemical reactions and syntheses of oxides. Chemical Society Reviews, 42(18), 7507–7520. https://doi.org/10.1039/c2cs35462d
Suzuki, K., Kato, T., Fuchida, S., & Tokoro, C. (2020). Removal mechanisms of cadmium by δ-MnO2 in adsorption and coprecipitation processes at pH 6. Chemical Geology, 550, 119744. https://doi.org/10.1016/j.chemgeo.2020.119744
Takacs, L. (2013). The historical development of mechanochemistry. Chemical Society Reviews, 42(18), 7649–7659. https://doi.org/10.1039/c2cs35442j
Tanong, K., Tran, L. H., Mercier, G., & Blais, J. F. (2017). Recovery of Zn (II), Mn (II), Cd (II) and Ni (II) from the unsorted spent batteries using solvent extraction, electrodeposition and precipitation methods. Journal of Cleaner Production, 148, 233–244. https://doi.org/10.1016/j.jclepro.2017.01.158
Waalkes, M. P. (2000). Cadmium carcinogenesis in review. Journal of Inorganic Biochemistry, 79(1–4), 241–244. https://doi.org/10.1016/S0162-0134(00)00009-X
Wang, Y., & Forssberg, E. (2006). Production of carbonate and silica nano-particles in stirred bead milling. International Journal of Mineral Processing, 81(1), 1–14. https://doi.org/10.1016/j.minpro.2006.05.007
Wang, W., Zhao, Y., Yi, H., Chen, T., Kang, S., Zhang, T., et al. (2019). Pb(ΙΙ) removal from water using porous hydrogel of chitosan-2D montmorillonite. International Journal of Biological Macromolecules, 128, 85–93. https://doi.org/10.1016/j.ijbiomac.2019.01.098
Xto, J. M., Borca, C. N., Van Bokhoven, J. A., & Huthwelker, T. (2019). Aerosol-based synthesis of pure and stable amorphous calcium carbonate. Chemical Communications, 55(72), 10725–10728. https://doi.org/10.1039/c9cc03749g
Zhang, T., Zhao, Y., Bai, H., Liu, Y., Liu, Y., & Zhang, Q. (2019a). Efficient As(III) removal directly as basic iron arsenite by in-situ generated Fe(III) hydroxide from ferrous sulfate on the surface of CaCO3. Applied Surface Science, 493, 569–576. https://doi.org/10.1016/j.apsusc.2019.07.048
Zhang, T., Zhao, Y., Kang, S., Li, Y., & Zhang, Q. (2019b). Formation of active Fe(OH)3 in situ for enhancing arsenic removal from water by the oxidation of Fe(II)in air with the presence of CaCO3. Journal of Cleaner Production, 227, 1–9. https://doi.org/10.1016/j.jclepro.2019.04.199
Zhang, C., Wu, L., Ma, J., Wang, M., Sun, J., & Waite, T. D. (2020). Evaluation of long-term performance of a continuously operated flow-electrode CDI system for salt removal from brackish waters. Water Research, 173, 115580. https://doi.org/10.1016/j.watres.2020.115580
Zhang, T., Wang, W., Zhao, Y., Bai, H., Wen, T., Kang, S., et al. (2021a). Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nano-composites. Chemical Engineering Journal, 420, 127574. https://doi.org/10.1016/j.cej.2020.127574
Zhang, T., Zhao, Y., Kang, S., Bai, H., & Gu, W. (2021b). Mechanical activation of zero-valent iron ( ZVI ) in the presence of CaCO 3: Improved reactivity of ZVI for enhancing As ( III ) removal from water. Journal of Cleaner Production, 286, 124926. https://doi.org/10.1016/j.jclepro.2020.124926
Zhang, T., Bai, H., Zhao, Y., Ren, B., Wen, T., Chen, L., et al. (2022). Precise cation recognition in two-dimensional nanofluidic channels of clay membranes imparted from intrinsic selectivity of clays. ACS Nano. https://doi.org/10.1021/acsnano.2c00866
Zhou, F., Ye, G., Gao, Y., Wang, H., Zhou, S., Liu, Y., & Yan, C. (2022). Cadmium adsorption by thermal-activated sepiolite: Application to in-situ remediation of artificially contaminated soil. Journal of Hazardous Materials, 423. https://doi.org/10.1016/j.jhazmat.2021.127104
Funding
The financial supports for this work from the Natural Science Foundation of Hubei Province of China (2021CFB554), Major Technical Innovation Project of Hubei Province (2021BEC022), the National Natural Science Foundation of China (Nos. 51904215 and 51874220), Hubei Key Laboratory of Mineral Resources Processing and Environment (Wuhan University of Technology) (No. ZHJJ202008), and the Fundamental Research Funds for the Central Universities (2020IVB016) are gratefully acknowledged. Haoyu Bai greatly acknowledges the financial support provided by China Scholarship Council (CSC)-Imperial Scholarship (CSC No. 202106950021).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Zhao, Y., Wen, T. et al. Efficient Cd(II) Removal from Aqueous Solution Using Mechanically Activated CaCO3: Removal Pathway and Mechanism. Water Air Soil Pollut 233, 378 (2022). https://doi.org/10.1007/s11270-022-05858-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05858-5