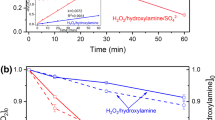
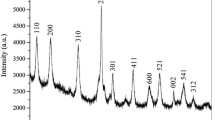
Abstract
In this study, pyrite/peroxymonosulfate (PMS)/hydroxylamine (HA) system could decolorize rhodamine B (RhB) in a wide pH range of 3.0-10.0. Near-100% decolorization of RhB was realized under optimum conditions of pyrite 0.4 g L−1, HA 0.8 mM, PMS 1.6 mM, and initial pH 4.0. Radical (mainly SO4•−) oxidation was the dominant process for RhB decolorization by pyrite/PMS/HA system, but nonradical (largely PMS) oxidation also played a non-negligible role. HA can act as a metal-free activator for PMS activation, and a reductant to boost Fe(III)/Fe(II) cycle in solution and on pyrite surface, which not only increased the production of radicals but also enhanced the stability of pyrite via inhibiting the S22− consumption. SO4•− that produced from pyrite oxidation was mainly from PMS activated by the Fe(II) in solution. Four other dye pollutants could also be decolorized completely. Our study suggests a promising process of pyrite/PMS/HA for organic dye pollutant treatment.







Similar content being viewed by others
References
Anipsitakis, G. P., & Dionysiou, D. D. (2004). Radical generation by the interaction of transition metals with common oxidants. Environmental Science & Technology, 38(13), 3705–3712.
Bae, S., Kim, D., & Lee, W. (2013). Degradation of diclofenac by pyrite catalyzed Fenton oxidation. Applied Catalysis B: Environmental, 134-135, 93–102.
Ball, D. L., & Edwards, J. O. (1956). The kinetics and mechanism of the decomposition of Caro’s acid. I. Journal of the American Chemical Society, 78(6), 1125–1129.
Banazadeh, A., Salimi, H., Khaleghi, M., & Shafiei-Haghighi, S. (2016). Highly efficient degradation of hazardous dyes in aqueous phase by supported palladium nanocatalyst─A green approach. Journal of Environmental Chemical Engineering, 4(2), 2178–2186.
Bokare, A. D., & Choi, W. (2014). Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. Journal of Hazardous Materials, 275, 121–135.
Brillas, E., & Martínez-Huitle, C. A. (2015). Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Applied Catalysis B: Environmental, 166-167, 603–643.
Buxton, G. V., Greenstock, C. L., Helman, W. P., & Ross, A. B. (1988). Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. Journal of Physical and Chemical Reference Data, 17(2), 513–886.
Buxton, G. V., Malone, T. N., & Arthur Salmon, G. (1997). Reaction of SO4•- with Fe2+, Mn2+ and Cu2+ in aqueous solution. Journal of the Chemical Society, Faraday Transactions, 93(16), 2893–2897.
Cai, Y., Pan, Y., Xue, J., Sun, Q., Su, G., & Li, X. (2009). Comparative XPS study between experimentally and naturally weathered pyrites. Applied Surface Science, 255(21), 8750–8760.
Chandra, A. P., & Gerson, A. R. (2010). The mechanisms of pyrite oxidation and leaching: A fundamental perspective. Surface Science Reports, 65(9), 293–315.
Chen, L., Ma, J., Li, X., Zhang, J., Fang, J., Guan, Y., & Xie, P. (2011). Strong enhancement on Fenton oxidation by addition of hydroxylamine to accelerate the ferric and ferrous iron cycles. Environmental Science & Technology, 45(9), 3925–3930.
Chen, L., Peng, X., Liu, J., Li, J., & Wu, F. (2012). Decolorization of orange II in aqueous solution by an Fe(II)/sulfite system: Replacement of persulfate. Industrial & Engineering Chemistry Research, 51(42), 13632–13638.
Chen, G., Nengzi, L. C., Li, B., Gao, Y., Zhu, G., & Cheng, X. (2019). Octadecylamine degradation through catalytic activation of peroxymonosulfate by FeMn layered double hydroxide. Science of The Total Environment, 695, 133963.
Diao, Z., Liu, J., Hu, Y., Kong, L., Jiang, D., & Xu, X. (2017). Comparative study of Rhodamine B degradation by the systems pyrite/H2O2 and pyrite/persulfate: Reactivity, stability, products and mechanism. Separation and Purification Technology, 184, 374–383.
Diao, Z., Lin, Z., Chen, X., Yan, L., Dong, F., Qian, W., Kong, L., Du, J., & Chu, W. (2020). Ultrasound-assisted heterogeneous activation of peroxymonosulphate by natural pyrite for 2,4-diclorophenol degradation in water: Synergistic effects, pathway and mechanism. Chemical Engineering Journal, 389, 123771.
Feng, Y., Wu, D., Deng, Y., Zhang, T., & Shih, K. (2016). Sulfate radical-mediated degradation of sulfadiazine by CuFeO2 rhombohedral crystal-catalyzed peroxymonosulfate: Synergistic effects and mechanisms. Environmental Science & Technology, 50(6), 3119–3127.
Feng, Y., Wu, D., Zhou, Y., & Shih, K. (2017). A metal-free method of generating sulfate radicals through direct interaction of hydroxylamine and peroxymonosulfate: Mechanisms, kinetics, and implications. Chemical Engineering Journal, 330, 906–913.
Feng, Y., Li, H., Lin, L., Kong, L., Li, X., Wu, D., Zhao, H., & Shih, K. (2018). Degradation of 1,4-dioxane via controlled generation of radicals by pyrite-activated oxidants: Synergistic effects, role of disulfides, and activation sites. Chemical Engineering Journal, 336, 416–426.
Ghanbari, F., & Martínez-Huitle, C. A. (2019). Electrochemical advanced oxidation processes coupled with peroxymonosulfate for the treatment of real washing machine effluent: A comparative study. Journal of Electroanalytical Chemistry, 847, 113182.
Ghanbari, F., & Moradi, M. (2017). Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chemical Engineering Journal, 310, 41–62.
Ghanbari, F., Khatebasreh, M., Mahdavianpour, M., & Lin, K. A. (2020). Oxidative removal of benzotriazole using peroxymonosulfate/ozone/ultrasound: Synergy, optimization, degradation intermediates and utilizing for real wastewater. Chemosphere, 244, 125326.
Giannakis, S., Lin, K.-Y. A., & Ghanbari, F. (2021). A review of the recent advances on the treatment of industrial wastewaters by sulfate radical-based advanced oxidation processes (SR-AOPs). Chemical Engineering Journal, 406, 127083.
Guan, Y. H., Ma, J., Li, X. C., Fang, J. Y., & Chen, L. W. (2011). Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system. Environmental Science & Technology, 45(21), 9308–9314.
Hassani, A., Eghbali, P., Kakavandi, B., Lin, K.-Y. A., & Ghanbari, F. (2020). Acetaminophen removal from aqueous solutions through peroxymonosulfate activation by CoFe2O4/mpg-C3N4 nanocomposite: Insight into the performance and degradation kinetics. Environmental Technology & Innovation, 20, 101127.
He, D. Q., Zhang, Y. J., Pei, D. N., Huang, G. X., Liu, C., Li, J., & Yu, H. Q. (2020). Degradation of benzoic acid in an advanced oxidation process: The effects of reducing agents. Journal of Hazardous Materials, 382, 121090.
Hickerson, R. P., Watkins-Sims, C. D., Burrows, C. J., Atkins, J. F., Gesteland, R. F., & Felden, B. (1998). A nickel complex cleaves uridine in folded RNA structures: Application to E. coli tmRNA and related engineered molecules. Journal of Molecular Biology, 279(3), 577–587.
Hou, X., Huang, X., Jia, F., Ai, Z., Zhao, J., & Zhang, L. (2017). Hydroxylamine promoted goethite surface Fenton degradation of organic pollutants. Environmental Science & Technology, 51(9), 5118–5126.
Hu, P., & Long, M. (2016). Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Applied Catalysis B: Environmental, 181, 103–117.
Javaid, R., & Qazi, U. Y. (2019). Catalytic oxidation process for the degradation of synthetic dyes: An overview. International Journal of Environmental Research and Public Health, 16(11), 2066–2093.
Ji, F., Zhang, H., Wei, X., Zhang, Y., & Lai, B. (2019). Efficient degradation of atrazine by Co-NZ catalyst prepared by electroless plating in the presence of peroxymonosulfate: Characterization, performance and mechanistic consideration. Chemical Engineering Journal, 359, 1316–1326.
Khabbaz, M., & Entezari, M. H. (2017). Degradation of diclofenac by sonosynthesis of pyrite nanoparticles. Journal of Environmental Management, 187, 416–423.
Lange, A., & Brauer, H. (1996). On the formation of dioxiranes and of singlet oxygen by the ketone-catalysed decomposition of Caro’s acid. Journal of the Chemical Society, Perkin Transactions 2, 5(5), 805–811.
Lei, Y., Chen, C., Ai, J., Lin, H., Huang, Y., & Zhang, H. (2016). Selective decolorization of cationic dyes by peroxymonosulfate: Non-radical mechanism and effect of chloride. RSC Advances, 6(2), 866–871.
Li, J., Wan, Y., Li, Y., Yao, G., & Lai, B. (2019). Surface Fe(III)/Fe(II) cycle promoted the degradation of atrazine by peroxymonosulfate activation in the presence of hydroxylamine. Applied Catalysis B: Environmental, 256, 117782.
Li, Y., Li, J., Pan, Y., Xiong, Z., Yao, G., Xie, R., & Lai, B. (2020). Peroxymonosulfate activation on FeCo2S4 modified g-C3N4 (FeCo2S4-CN): Mechanism of singlet oxygen evolution for nonradical efficient degradation of sulfamethoxazole. Chemical Engineering Journal, 384, 123361.
Li, T., Abdelhaleem, A., Chu, W., & Xu, W. (2021). Efficient activation of oxone by pyrite for the degradation of propanil: Kinetics and degradation pathway. Journal of Hazardous Materials, 403, 123930.
Liu, W., Wang, Y., Ai, Z., & Zhang, L. (2015). Hydrothermal synthesis of FeS2 as a high-efficiency Fenton reagent to degrade alachlor via superoxide-mediated Fe(II)/Fe(III) cycle. ACS Applied Materials & Interfaces, 7(51), 28534–28544.
Luan, J., Li, M., Ma, K., Li, Y., & Zou, Z. (2011). Photocatalytic activity of novel Y2InSbO7 and Y2GdSbO7 nanocatalysts for degradation of environmental pollutant rhodamine B under visible light irradiation. Chemical Engineering Journal, 167(1), 162–171.
Moses, C. O., & Herman, J. S. (1991). Pyrite oxidation at circumneutral pH. Geochimica et Cosmochimica Acta, 55(2), 471–482.
Neta, P., Huie, R. E., & Ross, A. B. (1988). Rate constants for reactions of inorganic radicals in aqueous solution. Journal of Physical and Chemical Reference Data, 17(3), 1027–1284.
Nidheesh, P. V., Gandhimathi, R., & Sanjini, N. S. (2014). NaHCO3 enhanced rhodamine B removal from aqueous solution by graphite–graphite electro Fenton system. Separation and Purification Technology, 132, 568–576.
Oral, O., & Kantar, C. (2019). Diclofenac removal by pyrite-Fenton process: Performance in batch and fixed-bed continuous flow systems. Science of The Total Environment, 664, 817–823.
Rastogi, A., Al-Abed, S. R., & Dionysiou, D. D. (2009). Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Applied Catalysis B: Environmental, 85(3-4), 171–179.
Rimstidt, J. D., & Vaughan, D. J. (2003). Pyrite oxidation: A state-of-the-art assessment of the reaction mechanism. Geochimica et Cosmochimica Acta, 67(5), 873–880.
Robinson, R. A., & Bower, V. E. (1961). The ionization constant of hydroxylamine. The Journal of Physical Chemistry, 65(7), 1279–1280.
Spiro, M. (1979). The standard potential of the peroxosulphate/sulphate couple. Electrochimica Acta, 24(3), 313–314.
Stemmler, A. J., & Burrows, C. J. (2001). Guanine versus deoxyribose damage in DNA oxidation mediated by vanadium(IV) and vanadium(V) complexes. Journal of Biological Inorganic Chemistry, 6(1), 100–106.
Tan, C., Xu, Q., Sheng, T., Cui, X., Wu, Z., Gao, H., & Li, H. (2020). Reactive oxygen species generation in FeOCl nanosheets activated peroxymonosulfate system: Radicals and non-radical pathways. Journal of Hazardous Materials, 398, 123084.
Todd, E. C., Sherman, D. M., & Purton, J. A. (2003). Surface oxidation of pyrite under ambient atmospheric and aqueous (pH = 2 to 10) conditions: Electronic structure and mineralogy from X-ray absorption spectroscopy. Geochimica et Cosmochimica Acta, 67(5), 881–893.
Wacławek, S., Lutze, H. V., Grübel, K., Padil, V. V. T., Černík, M., & Dionysiou, D. D. (2017). Chemistry of persulfates in water and wastewater treatment: A review. Chemical Engineering Journal, 330, 44–62.
Wang, S., Jia, Y., Song, L., & Zhang, H. (2018). Decolorization and mineralization of rhodamine B in aqueous solution with a triple system of Cerium(IV)/H2O2/Hydroxylamine. ACS Omega, 3(12), 18456–18465.
Xiao, S., Cheng, M., Zhong, H., Liu, Z., Liu, Y., Yang, X., & Liang, Q. (2020). Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chemical Engineering Journal, 384, 123265.
Xiong, Z., Zhang, H., Zhang, W., Lai, B., & Yao, G. (2019). Removal of nitrophenols and their derivatives by chemical redox: A review. Chemical Engineering Journal, 359, 13–31.
Yang, Z., Yu, A., Shan, C., Gao, G., & Pan, B. (2018). Enhanced Fe(III)-mediated Fenton oxidation of atrazine in the presence of functionalized multi-walled carbon nanotubes. Water Research, 137, 37–46.
Yin, R., Guo, W., Wang, H., Du, J., Zhou, X., Wu, Q., Zheng, H., Chang, J., & Ren, N. (2018). Selective degradation of sulfonamide antibiotics by peroxymonosulfate alone: Direct oxidation and nonradical mechanisms. Chemical Engineering Journal, 334, 2539–2546.
Zeng, L., Gong, J., Dan, J., Li, S., Zhang, J., Pu, W., & Yang, C. (2019). Novel visible light enhanced pyrite-Fenton system toward ultrarapid oxidation of p-nitrophenol: Catalytic activity, characterization and mechanism. Chemosphere, 228, 232–240.
Zhang, T., Zhu, H., & Croue, J. P. (2013). Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: Efficiency, stability, and mechanism. Environmental Science & Technology, 47(6), 2784–2791.
Zhang, J., Chen, M., & Zhu, L. (2016). Activation of peroxymonosulfate by iron-based catalysts for orange G degradation: Role of hydroxylamine. RSC Advances, 6(53), 47562–47569.
Zhou, Y., Wang, X., Zhu, C., Dionysiou, D. D., Zhao, G., Fang, G., & Zhou, D. (2018). New insight into the mechanism of peroxymonosulfate activation by sulfur-containing minerals: Role of sulfur conversion in sulfate radical generation. Water Research, 142, 208–216.
Zou, J., Ma, J., Chen, L., Li, X., Guan, Y., Xie, P., & Pan, C. (2013). Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting Fe(III)/Fe(II) cycle with hydroxylamine. Environmental Science & Technology, 47(20), 11685–11691.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 225 kb)
Rights and permissions
About this article
Cite this article
He, GJ., Zhong, DJ., Xu, YL. et al. Highly Efficient Rhodamine B Decolorization by Pyrite/Peroxymonosulfate/Hydroxylamine System. Water Air Soil Pollut 232, 141 (2021). https://doi.org/10.1007/s11270-021-05086-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05086-3