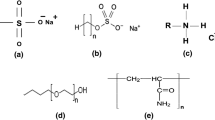
Abstract
The interfacial activity of dispersants can be enhanced by combining two or more surfactants to formulate the dispersant. This paper examines the effects of Bio-Saponin (BS), a phytogenic surfactant on the interfacial activity of synthetic dioctyl sulfosuccinate sodium salt (DOSS) usually adopted as a suitable surface-active agent in dispersants used in dealing with large-scale oil spills. The o/w emulsion created with the binary DOSS-BS was very stable and recorded the least average droplet size compared with that of DOSS only and BS only. Lower surface and interfacial tension values were also obtained from the DOSS-BS binary formulation. The dispersion effectiveness was also higher compared with that of DOSS and BS. However, they were dependent on the salinity and type of crude oil. These observations were attributed to the moderation of the interaction between the anionic head group of DOSS by the polysaccharide hydrophilic group of BS. The results revealed the potential application of DOSS-BS binary dispersant in oil spill remediation and in other processes that would require an effective emulsifier.













Similar content being viewed by others
References
Abramson, D. M., et al. (2010). Impact on children and families of the Deepwater Horizon oil spill: preliminary findings of the Coastal Population Impact Study.
Asadov, Z. H., Rahimov, R. A., & Salamova, N. V. (2012). Synthesis of animal fats ethylolamides, ethylolamide phosphates and their petroleum-collecting and dispersing properties. Journal of the American Oil Chemists' Society, 89(3), 505–511.
Athas, J. C., et al. (2014). An effective dispersant for oil spills based on food-grade amphiphiles. Langmuir, 30(31), 9285–9294.
Beyer, J., et al. (2016). Environmental effects of the Deepwater Horizon oil spill: a review. Marine Pollution Bulletin, 110(1), 28–51.
BiO-Botanica. (2017). Bio-Saponins: the natural surface active agent. [cited 2020 January 9]; Available from: http://www.bio-botanica.com/research-literature/.
Bombardelli, E., et al. (2002). Pharmaceutical and cosmetic formulations with antimicrobial activity, Google Patents.
Böttcher, S., & Drusch, S. (2017). Saponins—self-assembly and behavior at aqueous interfaces. Advances in Colloid and Interface Science, 243, 105–113.
Brochu, C., et al. (1986). Dispersion of crude oil in seawater: the role of synthetic surfactants. Oil and Chemical Pollution, 3(4), 257–279.
Dubansky, B., et al. (2013). Multitissue molecular, genomic, and developmental effects of the Deepwater Horizon oil spill on resident Gulf killifish (Fundulus grandis). Environmental Science & Technology, 47(10), 5074–5082.
Efavi, J. K., et al. (2017). Effect of magnesium and sodium salts on the interfacial characteristics of soybean lecithin dispersants. Industrial & Engineering Chemistry Research, 56(44), 12608–12620.
Etkin, D. S. (2001). Analysis of oil spill trends in the United States and worldwide. In International Oil Spill Conference. American Petroleum Institute.
George, A. J. (1965). Legal status and toxicity of saponins. Food and Cosmetics Toxicology, 3, 85–91.
Ghosh, P. (2004). Coalescence of air bubbles at air–water interface. Chemical Engineering Research and Design, 82(7), 849–854.
Güçlü-Üstündağ, Ö., & Mazza, G. (2007). Saponins: properties, applications and processing. Critical Reviews in Food Science and Nutrition, 47(3), 231–258.
Hodgson, T., & Woods, D. (1969). The effect of surfactants on the coalescence of a drop at an interface. II. Journal of Colloid and Interface Science, 30(4), 429–446.
Hostettmann, K., & Marston, A. (2005). Saponins. Cambridge: Cambridge University Press.
Inc, B.-B (2004). Bio-Saponins: the natural surface active agent. [cited 2019 27/03/2019]; Available from: http://www.bio-botanica.com/research-literature/.
Jin, J., et al. (2018). An efficient and environmental-friendly dispersant based on the synergy of amphiphilic surfactants for oil spill remediation. Chemosphere.
Karthik, S., et al. (2016). Quillaja saponin: a prospective emulsifier for the preparation of solid lipid nanoparticles. Colloids and Surfaces B: Biointerfaces, 147, 274–280.
Kleindienst, S., Paul, J. H., & Joye, S. B. (2015). Using dispersants after oil spills: impacts on the composition and activity of microbial communities. Nature Reviews Microbiology, 13(6), 388.
Knudsen, O., Hokstad, J. N., & Brandvik, P. J. (1997). Leaching of surfactants used in oil spill chemicals from the oil into the water phase. Trondheim: SINTEF Applied Chemistry.
Kujawinski, E. B., et al. (2011). Fate of dispersants associated with the Deepwater Horizon oil spill. Environmental Science & Technology, 45(4), 1298–1306.
Li, P., et al. (2016). Offshore oil spill response practices and emerging challenges. Marine Pollution Bulletin, 110(1), 6–27.
Madrigal, A. C. (2018). The world has never seen an oil spill like this. January 19, 2018 [cited 2018 October 17]; Available from: https://www.theatlantic.com/technology/archive/2018/01/the-oil-spill-that-wasnt/550820/.
Mapelli, F., et al. (2017). Biotechnologies for marine oil spill cleanup: indissoluble ties with microorganisms. Trends in Biotechnology, 35(9), 860–870.
Mitchell, F. M., & Holdway, D. A. (2000). The acute and chronic toxicity of the dispersants Corexit 9527 and 9500, water accommodated fraction (WAF) of crude oil, and dispersant enhanced WAF (DEWAF) to Hydra viridissima (green hydra). Water Research, 34(1), 343–348.
Myers, D. (2005). Surfactant science and technology. John Wiley & Sons.
Naser, H. A. (2013). Assessment and management of heavy metal pollution in the marine environment of the Arabian Gulf: a review. Marine Pollution Bulletin, 72(1), 6–13.
Nyankson, E., et al. (2014). Comparison of the effectiveness of solid and solubilized dioctyl sodium sulfosuccinate (DOSS) on oil dispersion using the baffled flask test, for crude oil spill applications. Industrial & Engineering Chemistry Research, 53(29), 11862–11872.
Nyankson, E., et al. (2015a). Surfactant-loaded halloysite clay nanotube dispersants for crude oil spill remediation. Industrial & Engineering Chemistry Research, 54(38), 9328–9341.
Nyankson, E., DeCuir, M. J., & Gupta, R. B. (2015b). Soybean lecithin as a dispersant for crude oil spills. ACS Sustainable Chemistry & Engineering, 3(5), 920–931.
Nyankson, E., Rodene, D., & Gupta, R. B. (2016a). Advancements in crude oil spill remediation research after the Deepwater Horizon oil spill. Water, Air, & Soil Pollution, 227(1), 29.
Nyankson, E., et al. (2016b). Interfacially active hydroxylated soybean lecithin dispersant for crude oil spill remediation. ACS Sustainable Chemistry & Engineering, 4(4), 2056–2067.
Owoseni, O., et al. (2014). Release of surfactant cargo from interfacially-active halloysite clay nanotubes for oil spill remediation. Langmuir, 30(45), 13533–13541.
Owoseni, O., et al. (2016). Interfacial adsorption and surfactant release characteristics of magnetically functionalized halloysite nanotubes for responsive emulsions. Journal of Colloid and Interface Science, 463, 288–298.
Pagureva, N., et al. (2016). Surface properties of adsorption layers formed from triterpenoid and steroid saponins. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 491, 18–28.
Pezeshki, S., et al. (2000). The effects of oil spill and clean-up on dominant US Gulf coast marsh macrophytes: a review. Environmental Pollution, 108(2), 129–139.
Silliman, B. R., et al. (2012). Degradation and resilience in Louisiana salt marshes after the BP–Deepwater Horizon oil spill. Proceedings of the National Academy of Sciences, 109(28), 11234–11239.
Stanimirova, R., et al. (2011). Surface rheology of saponin adsorption layers. Langmuir, 27(20), 12486–12498.
Thavorn, J., et al. (2014). Competitive surfactant adsorption of AOT and Tween 20 on gold measured using a quartz crystal microbalance with dissipation. Langmuir, 30(37), 11031–11039.
Venosa, A. D., King, D. W., & Sorial, G. A. (2002). The baffled flask test for dispersant effectiveness: a round robin evaluation of reproducibility and repeatability. Spill Science & Technology Bulletin, 7(5–6), 299–308.
Westermeyer, W. (1991). Oil spill response capabilities in the United States. Environmental Science & Technology, 25(2), 196–200.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nyankson, E., Agyei-Tuffour, B., Efavi, J.K. et al. Potential Application of Dioctyl Sodium Sulfosuccinate Salt (DOSS)–Saponin Binary Dispersant in Oil Spill Remediation: Synergistic Interaction Between DOSS and Saponin. Water Air Soil Pollut 231, 76 (2020). https://doi.org/10.1007/s11270-020-4445-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-4445-x