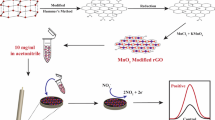
Abstract
Urea, being a nitrogen fertilizer, is crucial for plant growth but when excessively provided (above biuret 2% levels specified by the World Health Organization), plant characteristics are deeply affected. A real-time sensor to check the presence of excess urea in plants is therefore necessary. Towards this goal, a manganese oxide–reduced graphene oxide composite was synthesized by modified Hummer’s method and precipitation techniques, which was subsequently used as a nano-interface to immobilize urease enzyme for specific detection of urea. The synthesized nanocomposite helped in shuttling of electrons between the redox species and in enhanced electron transfer rate due to their high surface area, vindicated by their structural and morphological characterization using X-ray diffractometer (XRD), scanning electron microscope (SEM), and X-ray photoelectron spectrometer (XPS), and electrochemical characterization using cyclic voltammetry and amperometry, respectively. The fabricated biosensor for urea exhibited a linear range of 5–100 μM with a sensitivity of 9.7 × 10−3 μA μM−1, limit of detection of 14.693 μM, and a response time of 118 s.













Similar content being viewed by others
References
Augustin, M., Fenske, D., Bardenhagen, I., Westphal, A., Knipper, M., Plaggenborg, T., et al. (2015). Manganese oxide phases and morphologies: a study on calcination temperature and atmospheric dependence. Beilstein Journal of Nanotechnology, 6(1), 47–59. https://doi.org/10.3762/bjnano.6.6.
Biesinger, M. C., Payne, B. P., Grosvenor, A. P., Lau, L. W. M., Gerson, A. R., & Smart, R. S. C. (2011). Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, co and Ni. Applied Surface Science, 257(7), 2717–2730. https://doi.org/10.1016/j.apsusc.2010.10.051.
Bremner, J. M., & Krogmeier, M. J. (1989). Evidence that the adverse effect of urea fertilizer on seed germination in soil is due to ammonia formed through hydrolysis of urea by soil urease. Proceedings of the National Academy of Sciences of the United States of America, 86(November), 8185–8188.
Castillo-ortega, M. M., Rodriguez, D. E., Encinas, J. C., & Plascencia, M. (2002). Conductometric uric acid and urea biosensor prepared from electroconductive polyaniline–poly (n-butyl methacrylate) composites. Sensors and Actuators B: Chemical, 85(1–2), 19–25. https://doi.org/10.1016/s0925-4005(02)00045-x.
Chen, J., Gao, W., & Song, J. (2006). Flow-injection determination of iron ( III ) in soil by biamperometry using two independent redox couples. Sensors and Actuators B, 113, 194–200. https://doi.org/10.1016/j.snb.2005.02.047.
Congur, G., Eksin, E., & Erdem, A. (2019). Chitosan modified graphite electrodes developed for electrochemical monitoring of interaction between daunorubicin and DNA. Sensing and Bio-Sensing Research, 22(November 2018), 100255. https://doi.org/10.1016/j.sbsr.2018.100255.
Dervisevic, E., Dervisevic, M., Narkpa, J., & Mehmet, S. (2017). Sensors and actuators B: chemical development of novel amperometric urea biosensor based on Fc-PAMAM and MWCNT bio-nanocomposite film, 246, 920–926. https://doi.org/10.1016/j.snb.2017.02.122.
Drewniak, S., Muzyka, R., Stolarczyk, A., Pustelny, T., Kotyczka-Morańska, M., & Setkiewicz, M. (2016). Studies of reduced graphene oxide and graphite oxide in the aspect of their possible application in gas sensors. Sensors (Switzerland), 16(1). https://doi.org/10.3390/s16010103.
Ernandes, Ä. L. M. V. F. (2004). Enzymatic determination of urea in milk by sequential injection with spectrophotometric and conductometric detection, 6887–6890.
Francis, P. S., Lewis, S. W., & Lim, K. F. (2002). Analytical methodology for the determination of urea: current practice and future trends. Trends in Analytical Chemistry, 21(5), 389–400. https://doi.org/10.1016/S0165-9936(02)00507-1.
Food and Agriculture Organization of the United Nations. (2015). World Fertilizer Trends and Outlook to 2018. Rome: Food & Agriculture Organization of United Nations.
Fu, C., Zhao, G., Zhang, H., & Li, S. (2013). Evaluation and characterization of reduced graphene oxide nanosheets as anode materials for lithium-ion batteries. International Journal of Electrochemical Science, 8, 6269–6280. https://doi.org/10.1016/j.progpolymsci.2013.02.003.
Gabrovska, K., Ivanov, J., Vasileva, I., Dimova, N., & Godjevargova, T. (2011). International Journal of Biological Macromolecules Immobilization of urease on nanostructured polymer membrane and preparation of urea amperometric biosensor. International Journal of Biological Macromolecules, 48(4), 620–626. https://doi.org/10.1016/j.ijbiomac.2011.02.002.
Gautam, R. K., Bhattacharjee, H., Venkata Mohan, S., & Verma, A. (2016). Nitrogen doped graphene supported α-MnO2 nanorods for efficient ORR in a microbial fuel cell. RSC Advances, 6(111), 110091–110101. https://doi.org/10.1039/c6ra23392a.
Hao, W., Das, G., & Yoon, H. H. (2015). Fabrication of an amperometric urea biosensor using urease and metal catalysts immobilized by a polyion complex. Journal Of Electroanalytical Chemistry. https://doi.org/10.1016/j.jelechem.2015.03.015.
Holzwarth, U., & Gibson, N. (2011). The Scherrer equation versus the “Debye-Scherrer equation.”. Nature Nanotechnology, 6(9), 534. https://doi.org/10.1038/nnano.2011.145.
Hossain, M. F., & Park, J. Y. (2017). Fabrication of sensitive enzymatic biosensor based on multi-layered reduced graphene oxide added PtAu nanoparticles-modified hybrid electrode. PLoS One, 12(3), 1–16. https://doi.org/10.1371/journal.pone.0173553.
Hubalek, J., Hradecky, J., Adam, V., Krystofova, O., Huska, D., Masarik, M., et al. (2007). Spectrometric and voltammetric analysis of urease - nickel nanoelectrode as an electrochemical sensor. Sensors. https://doi.org/10.3390/s7071238.
Kojima, S., & Bohner, A. (2006). Molecular mechanisms of urea transport in plants, 91, 83–91. https://doi.org/10.1007/s00232-006-0868-6.
Kumar, S., Topkar, A., & Souza, S. F. D. (2008). Development of potentiometric urea biosensor based on urease immobilized in PVA – PAA composite matrix for estimation of blood urea nitrogen ( BUN )., 70, 1145–1150. https://doi.org/10.1016/j.jprot.2007.12.006.
Kyveryga, P. M., & Blackmer, A. M. (2018). Effects of soil pH on nitrification and losses of fall-applied anhydrous ammonia, 545–551. https://doi.org/10.31274/icm-180809-709.
Liang, A., Qin, H., Zhou, L., Zhang, Y., Ouyang, H., Wang, P., & Jiang, Z. (2011). A novel and sensitive resonance scattering assay for detection of urea in serum coupled urease catalytic reaction and NH4+ associated particle reaction. Bioprocess and Biosystems Engineering, 34(5), 639–645. https://doi.org/10.1007/s00449-011-0513-3.
López Pasquali, C. E., Fernández Hernando, P., & Durand Alegría, J. S. (2007). Spectrophotometric simultaneous determination of nitrite, nitrate and ammonium in soils by flow injection analysis. Analytica Chimica Acta, 600(1-2 SPEC. ISS), 177–182. https://doi.org/10.1016/j.aca.2007.03.015.
Majd, S. M., Salimi, A., & Astinchap, B. (2016). Manganese oxide nanoparticles / reduced graphene oxide as novel electrochemical platform for immobilization of FAD and its application as highly sensitive persulfate sensor, 493–502. https://doi.org/10.1002/elan.201500421.
Meibodi, A. S. E., & Haghjoo, S. (2014). Amperometric urea biosensor based on covalently immobilized urease on an electrochemically polymerized film of polyaniline containing MWCNTs. Synthetic Metals, 194, 1–6. https://doi.org/10.1016/j.synthmet.2014.04.009.
Mikkelsen, R. L. (1990). Biuret in urea fertilizer. Fertilizer Research, 26(1–3), 311–318. https://doi.org/10.1007/BF01048769.
Musil, M., Choi, B., & Tsutsumi, A. (2015). Morphology and electrochemical properties of α-, β-, γ-, and δ-MnO 2 synthesized by redox method. Journal of the Electrochemical Society, 162(10), A2058–A2065. https://doi.org/10.1149/2.0201510jes.
Nguyen, N. S., Das, G., & Hee, H. (2016). Biosensors and bioelectronics nickel / cobalt oxide-decorated 3D graphene nanocomposite electrode for enhanced electrochemical detection of urea., 77, 372–377. https://doi.org/10.1016/j.bios.2015.09.046.
Rafiq, K., Duy, H., Kyung, J., Min, J., Mi, B., Hum, C., & Yoo, H. (2017). Sensors and actuators B: chemical fabrication of a highly effective electrochemical urea sensing platform based on urease-immobilized silk fibroin scaffold and aminated glassy carbon electrode. Sensors and Actuators B: Chemical, 251, 472–480. https://doi.org/10.1016/j.snb.2017.05.048.
Rathnakumar, S. S., Noluthando, K., Kulandaiswamy, A. J., Rayappan, J. B. B., Kasinathan, K., Kennedy, J., & Maaza, M. (2019). Stalling behaviour of chloride ions: a non-enzymatic electrochemical detection of Α-Endosulfan using CuO interface. Sensors and Actuators, B: Chemical, 293(April), 100–106. https://doi.org/10.1016/j.snb.2019.04.141.
Rayanasukha, Y., Pratontep, S., Porntheeraphat, S., Bunjongpru, W., & Nukeaw, J. (2016). Surface & coatings technology non-enzymatic urea sensor usingmolecularly imprinted polymers surface modified based-on ion-sensitive field effect transistor ( ISFET ). Surface & Coatings Technology, 306, 147–150. https://doi.org/10.1016/j.surfcoat.2016.05.060.
Samdani, J., Samdani, K., Hoon, N., & Hee, J. (2017). Applied surface science a new protocol for the distribution of MnO 2 nanoparticles on rGO sheets and the resulting electrochemical performance. Applied Surface Science, 399, 95–105. https://doi.org/10.1016/j.apsusc.2016.12.086.
Saxena, A., Bhattacharya, A., Kumar, S., Epstein, I. R., & Sahney, R. (2017). Journal of Colloid and Interface Science Biopolymer matrix for nano-encapsulation of urease – a model protein and its application in urea detection. Journal of Colloid and Interface Science, 490, 452–461. https://doi.org/10.1016/j.jcis.2016.11.030.
Shrivastava, S., Jadon, N., & Jain, R. (2016). Trends in Analytical Chemistry Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: a review. Trends in Analytical Chemistry, 82, 55–67. https://doi.org/10.1016/j.trac.2016.04.005.
Shukla, S. K., Mishra, A. K., Mamba, B. B., & Arotiba, O. A. (2014). Enzyme and microbial technology zirconia-poly ( propylene imine ) dendrimer nanocomposite based electrochemical urea biosensor. Enzyme and Microbial Technology, 66, 48–55. https://doi.org/10.1016/j.enzmictec.2014.08.003.
Skoglund, S., Hedberg, J., Yunda, E., Godymchuk, A., Blomberg, E., & Odnevall Wallinder, I. (2017). Difficulties and flaws in performing accurate determinations of zeta potentials of metal nanoparticles in complex solutions - four case studies. PLoS One, 12(7), 1–19. https://doi.org/10.1371/journal.pone.0181735.
Witte, C. (2011). Plant science urea metabolism in plants. Plant Science, 180(3), 431–438. https://doi.org/10.1016/j.plantsci.2010.11.010.
Wu, Z., Li, C., Yu, J., & Chen, X. (2017). Sensors and actuators B : chemical MnO 2 / reduced graphene oxide nanoribbons : facile hydrothermal preparation and their application in amperometric detection of hydrogen peroxide. Sensors and Actuators B: Chemical, 239, 544–552. https://doi.org/10.1016/j.snb.2016.08.062.
Xu, C., Shi, X., Ji, A., Shi, L., Zhou, C., & Cui, Y. (2015). Fabrication and characteristics of reduced graphene oxide produced with different green reductants. https://doi.org/10.1371/journal.pone.0144842.
Zhou, C., Wang, J., Liu, X., Chen, F., Di, Y., Gao, S., & Shi, Q. (2018). Magnetic and thermodynamic properties of α, β, γ and δ-MnO 2. New Journal of Chemistry, 42(11), 8400–8407. https://doi.org/10.1039/C8NJ00896ES.
Acknowledgments
The authors are grateful to SERB, Department of Science and Technology, New Delhi, for their financial support (EMR/2016/001789 dated 13/01/2017).We also acknowledge SASTRA Deemed University, Thanjavur, for extending infrastructural support to carry out the study.
Funding
This study received financial support from SERB, Department of Science and Technology, New Delhi, (EMR/2016/001789 dated 13/01/2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramasami Sundhar Baabu, P., Gumpu, M.B., Nesakumar, N. et al. Electroactive Manganese Oxide–Reduced Graphene Oxide Interfaced Electrochemical Detection of Urea. Water Air Soil Pollut 231, 545 (2020). https://doi.org/10.1007/s11270-020-04899-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04899-y