Abstract
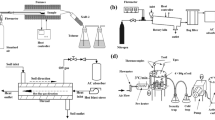
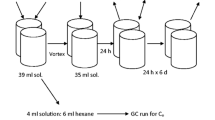
Thermal desorption (TD) is an effective way to remove hydrocarbons from soil. Soil exposure to temperatures between 100 and 400 °C to volatilize hydrocarbons can alter soil chemical properties that have potential to change phosphorus (P) dynamics. A series of laboratory soil sorption and desorption experiments were conducted on native and thermal desorption (TD)-treated soils to determine behavior of P with respect to availability for plant uptake and risk for runoff. The average relative increase in P sorption after TD treatment was between 14 and 26% as shown by Smax values. This increase in soil P sorption capacity is likely due to the potential creation of biochar materials and mineral transformations of Fe and Al oxides during TD treatment. Dissolved organic carbon association with P did not influence sorption. Desorption of P was least in TD-treated soils, indicating that the interaction between P and binding sites was strong. The amount of P retained by these soils (Smax values) may be of agronomic concern and should be considered when developing fertility plans. Based on these results, TD-treated soils pose no apparent threat to nearby surface waters.



Similar content being viewed by others
References
Auxtero, E., Madeira, M., & Sousa, E. (2008). Phosphorus sorption maxima and desorbability in selected soils with andic properties from the Azores, Portugal. Geoderma, 144, 535–544.
Bacelo, H., Pintor, A. M. A., Santos, S. C. R., Boaventura, R. A. R., & Botelho, C. M. S. (2020). Performance and prospects of different absorbents for phosphorus uptake and recovery from water. Chemical Engineering Journal, 381, 122566.
Bol, R., Julich, D., Brödlin, D., Siemens, J., Kaiser, K., Dippold, M. A., Spielvogel, S., Zilla, T., Mewes, D., von Blanckenburg, F., Puhlmann, H., Holzmann, S., Weiler, M., Amelung, W., Lang, F., Kuzyakov, Y., Feger, K. H., Gottselig, N., Klumpp, E., Missong, A., Winkelmann, C., Uhlig, D., Sohrt, J., von Wilpert, K., Wu, B., & Hagedorn, F. (2016). Dissolved and colloidal phosphorus fluxes in forest ecosystems—An almost blind spot in ecosystem research. Journal of Plant Nutrition and Soil Science, 179, 425–438.
Chintala, R., Schumacher, T. E., McDonald, L. M., Clay, D. E., Malo, D. D., Papiernik, S. K., Clay, S. A., & Julson, J. L. (2014). Phosphorus sorption and availability from biochars and soil/biochar mixtures. Clean Soil, Air, & Water, 42, 626–634.
DeLuca, T., MacKenzie, M. D., & Gundale, M. (2009). Biochar effects on soil nutrient transformations. In J. Lehmann & S. Joseph (Eds.), Biochar for environmental management: Science and tech. London: Earthscan.
Diphare, M., and E. Muzenda. (2014). Remediation of oil contaminated soils: A review. Intl’ Conf. Chem. Integr. Waste Manag. Environ. Eng. 180–185.
Donald, R. G., Anderson, D. W., & Stewart, J. W. B. (1993). Potential role of dissolved organic carbon in phosphorus transport in forested soils. Soil Science Society of America Journal, 57, 1611–1618.
do Nascimento, C. A. C., Pagliari, P. H., Faria, L. A., Vitti, G. C (2018). Phosphorus Mobility and Behavior in Soils Treated with Calcium, Ammonium, and Magnesium Phosphates. Soil Science Society of America Journal 82(3),622–631.
Edzwald, J. K., Toensing, D. C., & Leung, M. C. (1976). Phosphate adsorption reactions with clay minerals. Environmental Science & Technology, 10, 485–490.
Elrashidi, M. A., & O’Connor, G. A. (1982). Boron sorption and desorption in soils. Soil Science Society of America Journal, 46, 27–31.
Falciglia, P. P., Giustra, M. G., & Vagliasindi, F. G. A. (2011). Low-temperature thermal desorption of diesel polluted soil: Influence of temperature and soil texture on contaminant removal kinetics. Journal of Hazardous Materials, 185, 392–400.
Franzen, D.W. (2009). Fertilizing hard red spring wheat and durum. 712, 1–8.
Fox, T. R., & Comerford, N. B. (1990). Low molecular weight organic acids in selected forest soils of southeastern USA. Soil Science Society of America Journal, 54, 1139–1144.
Graetz, D. A., & Nair, V. D. (2009). Phosphorus sorption isotherm determination. In J. L. Kovar & G. M. Pierzynski (Eds.), Methods of phosphorus analysis for soils, sediments, residuals, and waters. Southern Coop. Ser. Bull. 408 (pp. 33–37). Blacksburg: Virginia Tech University.
Guppy, C. N., Menzies, N. W., Moody, P. W., & Blamey, F. P. C. (2005). Competitive sorption reactions between phosphorus and organic matter in soil: A review. Australian Journal of Soil Research, 43, 189–202.
Hamby, D. M. (1996). Site remediation techniques supporting environmental restoration activities—A review. Science of The Total Environment, 191, 203–224.
Holford, I. C. R., & Mattingly, G. E. G. (1976). Phosphate adsorption and plant availability of phosphate. Plant and Soil, 44, 377–389.
Holford, I. C. R. (1997). Soil phosphorus: Its measurement, and its uptake by plants. Australian Journal of Soil Research, 35, 227–239.
Hongthanat, N., Kovar, J. L., & Thompson, M. L. (2011). Sorption indices to estimate risk of soil phosphorus loss in the Rathbun Lake watershed, Iowa. Soil Science, 176, 237–244.
Jagadamma, S., Mayes, M. A., & Phillips, J. R. (2012). Selective sorption of dissolved organic carbon compounds by temperate soils. PLoS One, 7, e50434.
Koide, R. T., Petprakob, K., & Peoples, M. (2011). Quantitative analysis of biochar in field soil. Soil Biology and Biochemistry, 43, 1563–1568.
Kruse, J., Abraham, M., Amelung, W., Baum, C., Bol, R., Kühn, O., Lewandowski, H., Niederberger, J., Oelmann, Y., Rüger, C., Santner, J., Siebers, M., Siebers, N., Spohn, M., Vestergren, J., Vogts, A., & Leinweber, P. (2015). Innovative methods in soil phosphorus research: A review. Journal of Plant Nutrition and Soil Science, 178, 43–88.
Lair, G. J., Zehetner, F., Khan, Z. H., & Gerzabek, M. H. (2009). Phosphorus sorption-desorption in alluvial soils of a young weathering sequence at the Danube River. Geoderma, 149, 39–44.
Lilienfein, J., Qualls, R. G., Uselman, S. M., & Bridgham, S. D. (2004). Adsorption of dissolved organic and inorganic phosphorus in soils of a weathering chronosequence. Soil Science Society of America Journal, 68, 620–628.
Lim, M. W., Von Lau, E., & Poh, P. E. (2016). A comprehensive guide of remediation technologies for oil contaminated soil: Present works and future directions. Marine Pollution Bulletin, 109, 14–45.
Lopez-Hernandez, D., & Burnham, C. P. (1974). The effect of pH on phosphate adsorption in soils. Journal of Soil Science, 25, 207–216.
McAlexander, B. L., Krembs, F. J., & Cardeñosa Mendoza, M. (2015). Treatability testing for weathered hydrocarbons in soils: Bioremediation, soil washing, chemical oxidation, and thermal desorption. Soil and Sediment Contamination, 24, 882–897.
Mead, J. A. (1981). A comparison of the Langmuir, Freundlich and Temkin equations to describe phosphate adsorption properties of soils. Australian Journal of Soil Research, 19, 333–342.
Mehlich, A. (1984). Mehlich III soil test extractant: A modification of Mehlich II extractant. Communications in Soil Science and Plant Analysis, 15, 1409–1416.
O’Brien, P. L., DeSutter, T. M., Casey, F. X. M., Derby, N. E., & Wick, A. F. (2016). Implications of using thermal desorption to remediate contaminated agricultural soil: Physical characteristics and hydraulic processes. Journal of Environmental Quality, 45, 1430–1436.
O’Brien, P. L., DeSutter, T. M., Casey, F. X. M., Wick, A. F., & Khan, E. (2017). Wheat growth in soils treated by ex situ thermal desorption. Journal of Environmental Quality, 46, 897–905.
O’Brien, P. L., DeSutter, T. M., Ritter, S. S., Casey, F. X. M., Wick, A. F., Khan, E., & Matthees, H. L. (2017b). A large-scale soil-mixing process for reclamation of heavily disturbed soils. Ecological Engineering, 109, 84–91.
O’Brien, P. L., DeSutter, T. M., & Casey, F. X. M. (2018). Natural degradation of low-level petroleum hydrocarbon contamination under crop management. Journal of Soils and Sediments, 3, 1–7.
Olsen, S. R., Cole C. V., Watanabe F. S. & Dean L. A.. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U. S. Dept. of Ag. Circular No. 939.
Pal, S. K. (2011). Phosphorus sorption-desorption characteristics of soils under different land use patterns of eastern India. Archives of Agronomy and Soil Science, 57, 365–376.
Patton, C. J., & Kryskalla J. R., (2003). Methods of analysis by the U.S. geological survey national water quality laboratory-evaluation of alkaline persulfate digestion as an alternative to Kjeldahl digestion for determination of total and dissolved nitrogen and phosphorus in water. U.S. Geol. Survey. Water-Resources Investigations Report 03–4174.
Peng, Y., Sun, Y., Sun, R., Zhou, Y., Tang, D. C. W., & Chen, Q. (2019). Optimizing the synthesis of Fe/Al (Hydr)oxides-biochars to maximize phosphate removal via response surface model. Journal of Cleaner Production, 237, 117770.
Pierzynski, G.M., J.T. Sims, and G.F. Vance. (2005). Soil phosphorus and environmental quality in soils and environmental quality, Third ed. p. 185-234.
Raven, K. P., & Hossner, L. R. (1994). Sorption and desorption quantity-intensity parameters related to plant-available soil phosphorus. Soil Science Society of America Journal, 58, 405–410.
Ritter, S., DeSutter, T., O’Brien, P., Casey, F., Wick, A., Horsager, K., & Khan, E. (2017). Binary exchanges of calcium, magnesium, and potassium on thermally desorbed soil. Soil Science Society of America Journal, 81, 1088–1095.
Sharpley, A. N., Daniel, T., Sims, T., Lemunyon, J., Stevens, R., & Parry, R. (2003). Agricultural phosphorus and eutrophication, USDA-ARS report 149 (2nd ed.). Washington, DC: U.S. Govt. Printing Office.
Solis, P., & Torrent, J. (1989). Phosphate sorption by calcareous vertisols and inceptisols of Spain. Soil Science Society of America Journal 53(2),456–459.
Sui, Y., & Thompson, M. L. (2000). Phosphorus sorption, desorption, and buffering capacity in a biosolids-amended mollisol. Soil Science Society of America Journal, 64, 164–169.
Tan, K. H. (1998). Principles of soil chemistry. Third ed. rev. and expanded. M. Dekker, New York. p. 327–356.
Tatàno, F., Felici, F., & Mangani, F. (2013). Lab-scale treatability tests for the thermal desorption of hydrocarbon-contaminated soils. Soil and Sediment Contamination, 22, 433–456.
Tellinghuisen, J., & Bolster, C. H. (2010). Least-squares analysis of phosphorus soil sorption data with weighting from variance function estimation: A statistical case for the Freundlich isotherm. Environmental Science & Technology, 44, 5029–5034.
USDA–NRCS. (2015). Web soil survey. http://websoilsurvey.nrcs.usda.gov/ (accessed 24 January 2020).
USEPA. (1993). Method 365.1. Determination of phosphorus by semi-automated colorimetry [online]. Available at https://www.epa.gov/sites/production/files/2015-08/documents/method_365-1_1993.pdf [accessed 24 January 2020].
USEPA. (1994). Low-temperature thermal desorption. In How to evaluate alternative cleanup technologies for underground storage tank sites: A guide for corrective action plan reviewers. EPA 510/B/94/003. Washington, DC: USEPA.
Vidonish, J. E., Zygourakis, K., Masiello, C. A., Gao, X., Mathieu, J., & Alvarez, P. J. J. (2016). Pyrolytic treatment and fertility enhancement of soils contaminated with heavy hydrocarbons. Environmental Science & Technology, 50, 2498–2506.
Xu, G., Sun, J., Shao, H., & Chang, S. X. (2014). Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecological Engineering, 62, 54–60.
Yang, G. R., Hao, X. Y., Li, C. L., & Li, Y. M. (2014). Effect of land use on soil phosphorus sorption-desorption under intensive agricultural practices in plastic-film greenhouses. Pedosphere, 24, 367–377.
Zehetner, F., & Miller, W. P. (2006). Soil variations along a climatic gradient in an Andean agro-ecosystem. Geoderma, 137, 126–134.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Croat, S.J., DeSutter, T.M., Casey, F.X.M. et al. Phosphorus Sorption and Desorption in Soils Treated by Thermal Desorption. Water Air Soil Pollut 231, 216 (2020). https://doi.org/10.1007/s11270-020-04579-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04579-x