Abstract
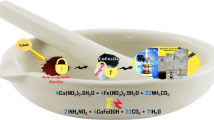
In the present work, chitosan/fluorapatite composite was successfully prepared and applied for the removal of chromium (VI). The synthesized materials were characterized using X-rays diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), scanning electronic microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDS). The thermogravimetric analysis (TGA) and pH of the point of zero charge (pHPZC) were also considered as a part of these characterizations. A batch system was carried out to evaluate the effects of contact time, initial Cr (VI) concentration, initial pH, and adsorbent dosage on the adsorption process. The regression coefficient value showed that the experimental data best fit to pseudo-second-order model (PSO), while the Langmuir adsorption isotherms best described the equilibrium adsorption data with highest qm of 81.34 and 100.92 mg/g for CS and CS-Fa, respectively. Finally, CS-Fa was successfully reused for more than 6 cycles without severe loss in its sorption capacity. The effect of various parameters such as pH, mass, temperature, and contact time was studied using response surface methodology (RSM) and the suggested optimized values by RSM were found to be 2.54 for pH, 25.75 °C, 36.63 min, and 86.72 mg of CS-Fa adsorbent. The maximum adsorption removal efficiency of Cr (VI) was equal to 91.28% under optimum conditions.








Similar content being viewed by others
References
Abatal, M., Olguin, M. T., Abdellaoui, Y., & Bouari, A. E. L. (2018). Sorption of Cd (II), Ni (II) and Zn (II) on natural, sodium-, and acid-modified clinoptilolite-rich tuff. Environment Protection Engineering, 44(1), 42–59. https://doi.org/10.5277/epe180104.
Abdel-Fattah, W. I., Sallam, A. S. M., Diab, A. M., & Ali, G. W. (2015). Tailoring the properties and functions of phosphate/silk/Ag/chitosan scaffolds. Materials Science and Engineering C, 54, 158–168. https://doi.org/10.1016/j.msec.2015.05.015.
Abdellaoui, Y., Olguin, M. T., Abatal, M., Bassam, A., & Giácoman-Vallejos, G. (2019). Relationship between Si/Al ratio and the sorption of Cd ( II ) by natural and modified clinoptilolite-rich tuff with sulfuric acid. Desalination and Water Treatment, 150, 157–165. https://doi.org/10.5004/dwt.2019.23792.
Abdellaoui, Y., Olguín, M. T., Abatal, M., Ali, B., Díaz Méndez, S. E., & Santiago, A. A. (2018). Comparison of the divalent heavy metals (Pb, Cu and Cd) adsorption behavior by montmorillonite-KSF and their calcium- and sodium-forms. Superlattices and Microstructures. https://doi.org/10.1016/j.spmi.2017.11.061.
Ait Ahsaine, H., El Alem, N., Anfar, Z., Ezahri, M., & Zbair, M. (2017). Acridine orange adsorption by zinc oxide/almond shell activated carbon composite: operational factors, mechanism and performance optimization using central composite design and surface modeling. Journal of Environmental Management, 206, 383–397. https://doi.org/10.1016/j.jenvman.2017.10.058.
Ait Ahsaine, H., Zbair, M., Anfar, Z., Naciri, Y., El haouti, R., El Alem, N., & Ezahri, M. (2018). Cationic dyes adsorption onto high surface area ‘almond shell’ activated carbon: Kinetics, equilibrium isotherms and surface statistical modeling. Materials Today Chemistry, 8, 121–132. https://doi.org/10.1016/j.mtchem.2018.03.004.
Aklil, A., Mouflih, M., & Sebti, S. (2004). Removal of heavy metal ions from water by using calcined phosphate as a new adsorbent. Journal of Hazardous Materials, 112(3), 183–190. https://doi.org/10.1016/j.jhazmat.2004.05.018.
Ali, G. W., El-Hotaby, W., Hemdan, B., & Abdel-Fattah, W. I. (2018). Thermosensitive chitosan/phosphate hydrogel-composites fortified with Ag versus Ag@Pd for biomedical applications. Life Sciences, 194, 185–195. https://doi.org/10.1016/j.lfs.2017.12.021.
Anfar, Z., Ait Ahsaine, H., Zbair, M., Amedlous, A., Ait El Fakir, A., Jada, A., & El Alem, N. (2019a). Recent trends on numerical investigations of response surface methodology for pollutants adsorption onto activated carbon materials: A review. Critical Reviews in Environmental Science and Technology, 1–42. https://doi.org/10.1080/10643389.2019.1642835.
Anfar, Z., Amedlous, A., Ait El Fakir, A., Ait Ahsaine, H., Zbair, M., Lhanafi, S., El Haouti, R., Jada, A., & El Alem, N. (2019b). Combined methane energy recovery and toxic dye removal by porous carbon derived from anaerobically modified digestate. ACS Omega, 4(5), 9434–9445. https://doi.org/10.1021/acsomega.9b00524.
Anfar, Z., El Haouti, R., Lhanafi, S., Benafqir, M., Azougarh, Y., & El Alem, N. (2017). Treated digested residue during anaerobic co-digestion of Agri-food organic waste: methylene blue adsorption, mechanism and CCD-RSM design. Journal of Environmental Chemical Engineering, 5(6), 5857–5867. https://doi.org/10.1016/j.jece.2017.11.015.
Anfar, Z., Zbair, M., Ahsaine, H. A., Abdellaoui, Y., El Fakir, A. A., Amaterz, E. H., Jada, A., & El Alem, N. (2019c). Preparation and characterization of porous carbon@ZnO-NPs for organic compounds removal: classical adsorption versus ultrasound assisted adsorption. ChemistrySelect, 4(17), 4981–4994. https://doi.org/10.1002/slct.201901043.
Anfar, Z., Zbair, M., Ahsaine, H. A., Ezahri, M., & Alem, N. E. (2018). Well-designed WO3/activated carbon composite for rhodamine B removal: synthesis, characterization, and modeling using response surface methodology. Fullerenes, Nanotubes, and Carbon Nanostructures, 26(6), 389–397. https://doi.org/10.1080/1536383X.2018.1440386.
Aydin, Y. A., & Aksoy, N. D. (2009). Adsorption of chromium on chitosan: optimization, kinetics and thermodynamics. Chemical Engineering Journal, 151(1–3), 188–194. https://doi.org/10.1016/j.cej.2009.02.010.
Chen, D., Li, W., Wu, Y., Zhu, Q., Lu, Z., & Du, G. (2013). Preparation and characterization of chitosan/montmorillonite magnetic microspheres and its application for the removal of Cr ( VI ). 221, 8–15. https://doi.org/10.1016/j.cej.2013.01.089.
Cimá-Mukul, C. A., Abdellaoui, Y., Abatal, M., Vargas, J., Santiago, A. A., & Barrón-Zambrano, J. A. (2019). Eco-efficient biosorbent based on Leucaena leucocephala residues for the simultaneous removal of Pb (II) and Cd (II) ions from water system: sorption and mechanism. Bioinorganic Chemistry and Applications, 2019, 1–13. https://doi.org/10.1155/2019/2814047.
Dessì, M., Borzacchiello, A., Mohamed, T. H. A., Abdel-Fattah, W. I., & Ambrosio, L. (2013). Novel biomimetic thermosensitive β-tricalcium phosphate/chitosan-based hydrogels for bone tissue engineering. Journal of Biomedical Materials Research - Part A, 101(10), 2984–2993. https://doi.org/10.1002/jbm.a.34592.
Dinker, M. K., & Kulkarni, P. S. (2015). Recent advances in silica-based materials for the removal of hexavalent chromium: a review. Journal of Chemical and Engineering Data, 60(9), 2521–2540. https://doi.org/10.1021/acs.jced.5b00292.
Elouahli, A., Zbair, M., Anfar, Z., Ahsaine, H. A., Khallok, H., Chourak, R., & Hatim, Z. (2018). Apatitic tricalcium phosphate powder: high sorption capacity of hexavalent chromium removal. Surfaces and Interfaces, 13, 139–147. https://doi.org/10.1016/j.surfin.2018.09.006.
Essamlali, Y., Amadine, O., Fihri, A., & Zahouily, M. (2019). Sodium modified fluorapatite as a sustainable solid bi-functional catalyst for biodiesel production from rapeseed oil. Renewable Energy, 133, 1295–1307. https://doi.org/10.1016/j.renene.2018.08.103.
Haffad, H., Zbair, M., Anfar, Z., Ahsaine, H. A., Bouhlal, H., & Khallok, H. (2019). Removal of reactive red-198 dye using chitosan as an adsorbent: optimization by central composite design coupled with response surface methodology. Toxin Reviews, 0(0), 1–13. https://doi.org/10.1080/15569543.2019.1584822.
Hameed, B. H., Ahmad, A. A., & Aziz, N. (2007). Isotherms, kinetics and thermodynamics of acid dye adsorption on activated palm ash. Chemical Engineering Journal, 133(1–3), 195–203. https://doi.org/10.1016/j.cej.2007.01.032.
Ho, Y. S., & Mckay, G. (1998). Kinetic models for the sorption of dye from aqueous solution by wood. In Trans IChemE, 76.
Huang, R., Yang, B., & Liu, Q. (2013). Removal of chromium (VI) ions from aqueous solutions with protonated crosslinked chitosan. Journal of Applied Polymer Science, 129(2), 908–915. https://doi.org/10.1002/app.38685.
Hughes, J. M., & Rakovan, J. (2002). The Crystal structure of apatite, Ca5(PO4)3(F,OH,Cl). Reviews in Mineralogy and Geochemistry, 48(1), 1–12. https://doi.org/10.2138/rmg.2002.48.1.
Kousalya, G. N., Rajiv Gandhi, M., & Meenakshi, S. (2010). Removal of toxic Cr (VI) ions from aqueous solution using nano-hydroxyapatite-based chitin and chitosan hybrid composites. Adsorption Science and Technology, 28(1), 49–64. https://doi.org/10.1260/0263-6174.28.1.49.
Lagergren, S. (1898). Zur theorie der sogenannten adsorption geloster stoffe. In Kungliga Svenska Vetenskapsakademiens (Handlingar, Vol. 24).
Langmuir, I. (1916). The constitution and fundamental properties of solids and liquids. Part I. Solids. Journal of the American Chemical Society, 38(11), 2221–2295. https://doi.org/10.1021/ja02268a002.
Li, L., Fan, L., Sun, M., Qiu, H., Li, X., Duan, H., & Luo, C. (2013). Adsorbent for hydroquinone removal based on graphene oxide functionalized with magnetic cyclodextrin-chitosan. International Journal of Biological Macromolecules, 58, 169–175. https://doi.org/10.1016/j.ijbiomac.2013.03.058.
Li, Y., Du, Q., Wang, X., Zhang, P., Wang, D., Wang, Z., & Xia, Y. (2010). Removal of lead from aqueous solution by activated carbon prepared from Enteromorpha prolifera by zinc chloride activation. Journal of Hazardous Materials, 183(1–3), 583–589. https://doi.org/10.1016/j.jhazmat.2010.07.063.
Liu, Q., Yang, B., Zhang, L., & Huang, R. (2015). Adsorptive removal of Cr (VI) from aqueous solutions by cross-linked chitosan/bentonite composite. Korean Journal of Chemical Engineering, 32(7), 1314–1322. https://doi.org/10.1007/s11814-014-0339-1.
Mobasherpour, I., Salahi, E., & Pazouki, M. (2011). Removal of nickel (II) from aqueous solutions by using nano-crystalline calcium hydroxyapatite. Journal of Saudi Chemical Society, 15(2), 105–112. https://doi.org/10.1016/j.jscs.2010.06.003.
Nan, N., Zhu, Y., & Han, Y. (2019). Flotation performance and mechanism of α-Bromolauric acid on separation of hematite and fluorapatite. Minerals Engineering, 132(September 2018), 162–168. https://doi.org/10.1016/j.mineng.2018.11.048.
Ouasfi, N., Zbair, M., Bouzikri, S., Anfar, Z., Bensitel, M., Ait Ahsaine, H., Sabbar, E., & Khamliche, L. (2019). Selected pharmaceuticals removal using algae derived porous carbon: experimental, modeling and DFT theoretical insights. RSC Advances, 9(17), 9792–9808. https://doi.org/10.1039/C9RA01086F.
Pal, P., & Pal, A. (2019). Treatment of real wastewater: kinetic and thermodynamic aspects of cadmium adsorption onto surfactant-modified chitosan beads. International Journal of Biological Macromolecules, 131, 1092–1100. https://doi.org/10.1016/j.ijbiomac.2019.03.121.
Panda, L., Das, B., & Rao, D. S. (2011). Studies on removal of lead ions from aqueous solutions using iron ore slimes as adsorbent. Korean Journal of Chemical Engineering, 28(10), 2024–2032. https://doi.org/10.1007/s11814-011-0094-5.
Rojas, G., Silva, J., Flores, J. A., Rodriguez, A., Ly, M., & Maldonado, H. (2005). Adsorption of chromium onto cross-linked chitosan. Separation and Purification Technology, 44(1), 31–36. https://doi.org/10.1016/j.seppur.2004.11.013.
Salgado-gómez, N., Macedo-miranda, M. G., & Olguín, M. T. (2014). Chromium VI adsorption from sodium chromate and potassium dichromate aqueous systems by hexadecyltrimethylammonium-modified zeolite-rich tuff. Applied Clay Science. https://doi.org/10.1016/j.clay.2014.04.013.
de Sampaio, C., G., Frota, L. S., Magalhães, H. S., Dutra, L. M. U., Queiroz, D. C., Araújo, R. S., Becker, H., de Souza, J. R. R., Ricardo, N. M. P. S., & Trevisan, M. T. S. (2015). Chitosan/mangiferin particles for Cr (VI) reduction and removal. International Journal of Biological Macromolecules, 78, 273–279. https://doi.org/10.1016/j.ijbiomac.2015.03.038.
Sessarego, S., Rodrigues, S. C. G., Xiao, Y., Lu, Q., & Hill, J. M. (2019). Phosphonium-enhanced chitosan for Cr (VI) adsorption in wastewater treatment. Carbohydrate Polymers, 211(Vi), 249–256. https://doi.org/10.1016/j.carbpol.2019.02.003.
Sethy, T. R., & Sahoo, P. K. (2019). Highly toxic Cr (VI) adsorption by (chitosan-g-PMMA)/silica bionanocomposite prepared via emulsifier-free emulsion polymerisation. International Journal of Biological Macromolecules, 122, 1184–1190. https://doi.org/10.1016/j.ijbiomac.2018.09.069.
Sivakumar, M., Manjubala, I., & Panduranga Rao, K. (2002). Preparation, characterization and in-vitro release of gentamicin from coralline hydroxyapatite-chitosan composite microspheres. Carbohydrate Polymers, 49(3), 281–288. https://doi.org/10.1016/S0144-8617(01)00331-9.
Song, C., Yu, H., Zhang, M., Yang, Y., & Zhang, G. (2013). Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. International Journal of Biological Macromolecules, 60, 347–354. https://doi.org/10.1016/j.ijbiomac.2013.05.039.
Streimikiene, D. (2015). Environmental indicators for the assessment of quality of life. Intellectual Economics, 9(1), 67–79. https://doi.org/10.1016/j.intele.2015.10.001.
Sun, Z., Chen, D., Chen, B., Kong, L., & Su, M. (2018). Enhanced uranium (VI) adsorption by chitosan modified phosphate rock. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 547(February), 141–147. https://doi.org/10.1016/j.colsurfa.2018.02.043.
Tsai, W. T., & Chang, C. Y. (1995). Surface characterization and thermodynamics of adsorption of methylene chloride on activated carbons. Journal of Environmental Science and Health. Part A: Environmental Science and Engineering and Toxicology, 30(3), 525–535. https://doi.org/10.1080/10934529509376215.
U., F (1906). Die adsorption in lusungen (Vol. 57, Issue 0, pp. 385–470). Journal of Physical Chemistry.
Uddin, M. K. (2017). A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chemical Engineering Journal, 308, 438–462. https://doi.org/10.1016/j.cej.2016.09.029.
Weber, W. J., & Morris, J. C. (1963). Kinetics of adsorption on carbon from solutions. Journal of the Sanitary Engineering Division, 89, 31–60.
Wu, Y., Zhang, S., Guo, X., & Huang, H. (2008). Adsorption of chromium (III) on lignin. Bioresource Technology, 99(16), 7709–7715. https://doi.org/10.1016/j.biortech.2008.01.069.
Xiao, Y., Liang, H., Chen, W., & Wang, Z. (2013). Applied surface science synthesis and adsorption behavior of chitosan-coated MnFe 2 O 4 nanoparticles for trace heavy metal ions removal. Applied Surface Science, 285, 498–504. https://doi.org/10.1016/j.apsusc.2013.08.083.
Yu, P., Wang, H., Bao, R., Liu, Z., Yang, W., Xie, B., & Yang, M. (2017). Self-assembled sponge-like chitosan/reduced graphene oxide/montmorillonite composite hydrogels without cross-linking of chitosan for effective Cr (VI) sorption. Vi. https://doi.org/10.1021/acssuschemeng.6b02254.
Zbair, M., Ahsaine, H. A., Anfar, Z., & Slassi, A. (2019). Carbon microspheres derived from walnut shell: rapid and remarkable uptake of heavy metal ions, molecular computational study and surface modeling. Chemosphere, 231, 140–150. https://doi.org/10.1016/j.chemosphere.2019.05.120.
Zbair, M., Ait Ahsaine, H., & Anfar, Z. (2018). Porous carbon by microwave assisted pyrolysis: an effective and low-cost adsorbent for sulfamethoxazole adsorption and optimization using response surface methodology. Journal of Cleaner Production. https://doi.org/10.1016/j.jclepro.2018.08.155.
Zhang, L., Luo, H., Liu, P., Fang, W., & Geng, J. (2016). A novel modified graphene oxide/chitosan composite used as an adsorbent for Cr (VI) in aqueous solutions. International Journal of Biological Macromolecules, 87, 586–596. https://doi.org/10.1016/j.ijbiomac.2016.03.027.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM1
(DOCX 155 kb)
Rights and permissions
About this article
Cite this article
Billah, R.E.K., Abdellaoui, Y., Anfar, Z. et al. Synthesis and Characterization of Chitosan/Fluorapatite Composites for the Removal of Cr (VI) from Aqueous Solutions and Optimized Parameters. Water Air Soil Pollut 231, 163 (2020). https://doi.org/10.1007/s11270-020-04535-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04535-9