Abstract
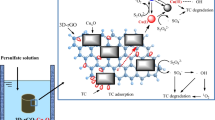
A composite catalyst of MCM-41-supported graphene oxide (GO) and iron (GO-MCM-Fe) was synthesized with solvothermal method and shown for the first time to be an efficient peroxydisulfate (PDS) activator to remove levofloxacin hydrochloride (LVF) in water. A total of 97.10% of LVF were removed within 10 min in the GO-MCM-Fe/PDS system. And the apparent rate constant of the GO-MCM-/Fe/PDS system was 11.78 and 1.35 times that of the GO-MCM-Fe system and MCM-Fe/PDS system, respectively. GO-MCM-Fe catalyst achieved lower iron ions (0.27%) than MCM-Fe (0.89%) at 60 min. Although GO could strengthen stability of the catalyst; this effect was negatively related with GO dosages in this work. And 5%wt Fe and 30 mL GO solution were chosen as the optimal condition for catalyst synthesis. The GO-MCM-Fe catalyst could be reused and the LVF removal ratio reached about 86% even after the fifth run. Both SO4−· and ·OH played the important roles and the latter was a dominant role. Additionally, in the process of GO-MCM-Fe activating PDS, there existed a redox interaction between the C=C of GO and Fe3+, in which the C=C of GO was oxidized into C=O; meanwhile, Fe3+ was reduced to Fe2+ which further activated PDS to generate radicals. This study provides a promising catalyst in effectively activating PDS to remove organic pollutants in water.








Similar content being viewed by others
References
Anipsitakis, G. P., & Dionysiou, D. D. (2003). Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environmental Science & Technology, 37, 4790–4797.
Anipsitakis, G. P., & Dionysiou, D. D. (2004). Radical generation by the interaction of transition metals with common oxidants. Environmental Science & Technology, 38, 3705–3712.
Bekris, L., Frontistis, Z., Trakakis, G., Sygellou, L., Galiotis, C., & Mantzavinos, D. (2017). Graphene: a new activator of sodium persulfate for the advanced oxidation of parabens in water. Journal of Environmental Management, 195, 125–132.
Carvalho, W. A., Wallau, M., & Schuchardt, U. (1999). Iron and copper immobilised on mesoporous MCM-41 molecular sieves as catalysts for the oxidation of cyclohexane. Journal of Molecular Catalysis A: Chemical, 144, 91–99.
Dong, H., Wei, M., Li, J., Fang, J., Gao, L., Li, X., & Xu, A. (2016). Catalytic performance of supported g-C3N4 on MCM-41 in organic dye degradation with peroxymonosulfate. RSC Advances, 6, 70747–70755.
Duan, X., Sun, H., Kang, J., Wang, Y., Indrawirawan, S., & Wang, S. (2015). Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons. ACS Catalysis, 5, 4629–4636.
Duan, X., Su, C., Zhou, L., Sun, H., Suvorova, A., Odedairo, T., Zhu, Z., Shao, Z., & Wang, S. (2016). Surface controlled generation of reactive radicals from persulfate by carbocatalysis on nanodiamonds. Applied Catalysis. B, Environmental, 194, 7–15.
Enoki, T., Fujii, S., & Takai, K. (2012). Zigzag and armchair edges in graphene. Carbon., 50, 3141–3145.
Esplugas, S., Bila, D. M., Krause, L. G. T., & Dezotti, M. (2007). Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. Journal of Hazardous Materials, 149, 631–642.
Fang, G., Wu, W., Deng, Y., & Zhou, D. (2017). Homogenous activation of persulfate by different species of vanadium ions for PCBs degradation. Chemical Engineering Journal, 323, 84–95.
Frank, B., Zhang, J., Blume, R., Schlogl, R., & Su, D. S. (2009). Heteroatoms increase the selectivity in oxidative dehydrogenation reactions on nanocarbons. Angewandte Chemie, 48, 6913–6917.
Geim, A. K., Novoselov, K. S., et al. (2007). The rise of graphene. Nature Materials, 6, 183–191.
Ghauch, A., Tuqan, A., et al. (2012). Oxidation of bisoprolol in heated persulfate/H2O systems: kinetics and products. Chemical Engineering Journal, 183, 162–171.
Ghauch, A., Tuqan, A. M., & Kibbi, N. (2015). Naproxen abatement by thermally activated persulfate in aqueous systems. Chemical Engineering Journal, 279, 861–873.
Han, D. H., Wan, J. Q., Ma, Y. W., Wang, Y., Li, Y., Li, D., & Guan, Z. Y. (2015). New insights into the role of organic chelating agents in Fe(II) activated persulfate processes. Chemical Engineering Journal, 269, 425–433.
Hu, M., Yao, Z., & Wang, X. (2017). Graphene-based nanomaterials for catalysis. Industrial and Engineering Chemistry Research, 56, 3477–3502.
Hussain, I., Zhang, Y., Huang, S., & Gao, Q. (2015). Degradation of p-chloroaniline by FeO3-xH3-2x/Fe0 in the presence of persulfate in aqueous solution. RSC Advances, 5(41), 079–41,087.
Hussain, I., Li, M., Zhang, Y., Li, Y., Huang, S., Xiaodong, D., Liu, G., Hayat, W., & Anwar, N. (2017). Insights into the mechanism of persulfate activation with nZVI/BC nanocomposite for the degradation of nonylphenol. Chemical Engineering Journal, 311, 163–172.
Jiang, D.-e., Sumpter, B. G., & Dai, S. (2007). Unique chemical reactivity of a graphene nanoribbon’s zigzag edge. The Journal of Chemical Physics, 126, 134701.
Jing, S., & Zhang, J. (2008). Determination of levofloxacin hydrochloride tablets by UV spectrophotometry. Progress in Pharmaceutical Science., 32, 323–325.
Kresge, C. T., Leonowicz, M. E., Roth, W. J., Vartuli, J. C., & Beck, J. S. (1992). Ordered mesoporous molecular sieves synthesized by a liquid-crystal tamplate mechanism. Nature., 359, 710–712.
Lee, G., Cho, K., et al. (2009). Electronic structures of zigzag graphene nanoribbons with edge hydrogenation and oxidation. Physical Review B, 79, 165440.
Liu, H. Z., Bruton, T. A., Doyle, F. M., & Sedlak, D. L. (2014). In situ chemical oxidation of contaminated groundwater by persulfate: decomposition by Fe(III)-and Mn (IV)-containing oxides and aquifer materials. Environmental Science & Technology, 48, 10,330–10,336.
Loh, K. P., Bao, Q., Ang, P. K., & Yang, J. (2010). The chemistry of graphene. Journal of Materials Chemistry, 20, 2277–2289.
Olmez-Hanci, T., Arslan-Alaton, I., Gurmen, S., Gafarli, I., Khoei, S., Safaltin, S., & Ozcelik, D. Y. (2018). Oxidative degradation of Bisphenol A by carbocatalytic activation of persulfate and peroxymonosulfate with reduced graphene oxide. Journal of Hazardous Materials, 360, 141–149.
Park, C. M., Heo, J., Wang, D., Su, C., & Yoon, Y. (2018). Heterogeneous activation of persulfate by reduced grapheme oxide–elemental silver/magnetite nanohybrids for the oxidative degradation of pharmaceuticals and endocrine disrupting compounds in water. Applied Catalysis B: Environmental, 225, 91–99.
Pi, Y., Ma, L., Zhao, P., Cao, Y., Gao, H., Wang, C., Li, Q., Dong, S., & Sun, J. (2018). Facile green synthetic graphene-based Co-Fe Prussian blue analogues as an activator of peroxymonosulfate for the degradation of levofloxacin hydrochloride. Journal of Colloid and Interface Science, 526, 18–27.
Rath, H., Dash, P., Singh, U. P., Avasthi, D. K., Kanjilal, D., & Mishra, N. C. (2015). Modification of the microstructure and electronic properties of rutile TiO2 thin films with 79 MeV Br ion irradiation. Nuclear Instruments and Methods in Physics Research B, 365, 553–559.
Saputra, E., Muhammad, S., Sun, H., Ang, H. M., Tadé, M. O., & Wang, S. (2013). Different crystallographic one-dimensional MnO2 nanomaterials and their superior performance in catalytic phenol degradation. Environmental Science & Technology, 47, 5882–5887.
Snyder, S. A., Westerhoff, P., Yoon, Y., & Sedlak, D. L. (2003). Pharmaceuticals, personal care products, and endocrine disruptors in water: implications for the water industry. Environmental Engineering Science, 20, 449–469.
Sun, B., Sato, M., & Clements, J. S. (2000). Oxidative processes occurring when pulsed high voltage discharges degrade phenol in aqueous solution. Environmental Science & Technology, 34, 509–513.
Sun, H. Q., Liu, S. Z., Zhou, G. L., Ang, H. M., Tade, M. O., & Wang, S. B. (2012). Reduced graphene oxide for catalytic oxidation of aqueous organic pollutants. ACS Applied Materials & Interfaces, 4, 5466–5471.
Sun, H., Liu, S., Liu, S., & Wang, S. (2014). A comparative study of reduced graphene oxide modified TiO2, ZnO and Ta2O5 in visible light photocatalytic/photochemical oxidation of methylene blue. Applied Catalysis B: Environmental, 146, 162–168.
Tamura, H., Goto, K., Yotsuyanagi, T., & Nagayama, M. (1974). Spectrophotometric determination of iron(II) with 1,10-phenanthroline in the presence of large amounts of iron(III). Talanta., 21, 314–318.
Thangavel, S., Raghavan, N., Kadarkarai, G., Kim, S.-J., & Venugopal, G. (2015). Graphene-oxide (GO)–Fe3+ hybrid nanosheets with effective sonocatalytic degradation of Reactive Red 120 and study of their kinetics mechanism. Ultrasonics Sonochemistry, 24, 123–131.
Wang, Y., Sun, H., Ang, H. M., Tadé, M. O., & Wang, S. (2015). 3D-hierarchically structured MnO2 for catalytic oxidation of phenol solutions by activation of peroxymonosulfate: structure dependence and mechanism. Applied Catalysis. B, Environmental, 164, 159–167.
Wang, J., Wang, S., et al. (2018). Activation of persulfate(PS)and peroxymonosulfate(PMS) and application for the degradation of emerging contaminants. Chemical Engineering Journal, 334, 1502–1517.
Xu, L. J., Chu, W., Gan, L., et al. (2015). Environmental application of graphene-based CoFe2O4 as an activator of peroxymonosulfate for the degradation of a plasticizer. Chemical Engineering Journal, 263, 435–443.
Xu, Y., Lin, H., Li, Y., Zhang, H., et al. (2017). The mechanism and efficiency of MnO2 activated persulfate process coupled with electrolysis. Science of the Total Environment, 609, 644–654.
Yang, H., Yan, R., Chen, H., Lee, D. H., & Zheng, C. (2007). Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel., 86, 1781–1788.
Zhang, B., Song, J., Yang, G., et al. (2014). Large-scale production of hi GO-quality graphene using glucose and ferric chloride. Chemical Science, 5, 4656–4660.
Zhu, Y., Murali, S., Cai, W., Li, X., Suk, J. W., Potts, J. R., & Ruoff, R. S. (2010). Graphene and graphene oxide: synthesis. Properties and Applications. Advanced Materials, 22, 3906–3924.
Funding
This study was supported by program (201809).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 786 kb)
Rights and permissions
About this article
Cite this article
Zuo, W., Wang, X., Zhang, D. et al. Performance and Mechanism of GO-MCM-Fe Composite Catalyst Activating Persulfate to Remove Levofloxacin Hydrochloride in Water. Water Air Soil Pollut 230, 255 (2019). https://doi.org/10.1007/s11270-019-4303-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4303-x