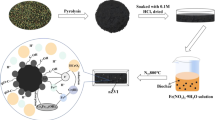
Abstract
Carboxymethyl cellulose–stabilized sulfidated nano zerovalent iron (CMC-S-nZVI) was tested for its capacity to the removal of Cr(VI) in this study. The effect of synthesis approaches on morphology and properties of CMC-S-nZVI was studied. Results revealed CMC-S-nZVI prepared by the surface corrosion method had favorable homogeneity and corrosion resistance. The structure and morphology of CMC-S-nZVI particles were investigated by transmission electron microscopy, X-ray powder diffraction, and Fourier-transform infrared spectrometry. Batch experiments showed that the removal efficiency of Cr(VI) by the CMC-S-nZVI particles was influenced by the S/Fe molar ratio, initial pH, initial Cr(VI) concentration, and the reaction temperature. Increasing S/Fe molar ratio from 0 to 0.35 enhanced Cr(VI) removal efficiency from 65.37 to 85.08%. Reducing pH value and improving the reaction temperature have a positive impact on Cr(VI) removal. The removal amount was 535 mg/g (total iron) CMC-S-nZVI with initial Cr(VI) concentration of 50 mg/L. Compared with CMC-nZVI, CMC-S-nZVI had better performance in Cr(VI) removal in a simulated groundwater system. The results indicated that CMC-S-nZVI might be applicable for in situ treatment of the Cr(VI)-containing groundwater.










Similar content being viewed by others
References
Alidokht, L., Khataee, A. R., Reyhanitabar, A., & Oustan, S. (2011). Reductive removal of Cr(VI) by starch-stabilized Fe 0 nanoparticles in aqueous solution. Desalination, 270(1), 105–110.
Choi, J., Choi, K., & Lee, W. (2009). Effects of transition metal and sulfide on the reductive dechlorination of carbon tetrachloride and 1,1,1-trichloroethane by FeS. Journal of Hazardous Materials, 162(2), 1151–1158.
Cirtiu, C. M., Raychoudhury, T., Ghoshal, S., & Moores, A. (2011). Systematic comparison of the size, surface characteristics and colloidal stability of zero valent iron nanoparticles pre- and post-grafted with common polymers. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 390(1), 95–104.
Deng, X., Qi, L., & Zhang, Y. (2018). Experimental study on adsorption of hexavalent chromium with microwave-assisted alkali modified fly ash. Water, Air, and Soil Pollution, 229(1), 18.
Dhal, B., Thatoi, H. N., Das, N. N., & Pandey, B. D. (2013). ChemInform abstract: Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. Journal of Hazardous Materials, 250-251(30), 272–291.
Du, J., Bao, J., Lu, C., & Werner, D. (2016). Reductive sequestration of chromate by hierarchical FeS@Fe(0) particles. Water Research, 102, 73–81.
Fan, D., Chen, S., Johnson, R. L., & Tratnyek, P. G. (2015). Field deployable chemical redox probe for quantitative characterization of carboxymethyl cellulose modified nano zerovalent iron. Environmental Science & Technology, 49(17), 10589–10597.
Fan, D., Johnson, G. O. B., Tratnyek, P. G., & Johnson, R. L. (2016a). Sulfidation of nano zerovalent iron (nZVI) for improved selectivity during in-situ chemical reduction (ISCR). Environmental Science & Technology, 50(17), 9558–9565.
Fan, D., O’Carroll, D. M., Elliott, D. W., Xiong, Z., Tratnyek, P. G., Johnson, R. L., et al. (2016b). Selectivity of nano zerovalent iron in in situ chemical reduction: Challenges and improvements. Remediation Journal, 26(4), 27–40.
Flora, S. D., Bagnasco, M., Serra, D., & Zanacchi, P. (1990). Genotoxicity of chromium compounds. A review. Mutation Research, Fundamental and Molecular Mechanisms of Mutagenesis, 238(2), 99–172.
Gheju, M. (2011). Hexavalent chromium reduction with zero-valent Iron (ZVI) in aquatic systems. Water, Air, and Soil Pollution, 222(1–4), 103–148.
Greenlee, L. F., Torrey, J. D., Amaro, R. L., & Shaw, J. M. (2012). Kinetics of zero valent iron nanoparticle oxidation in oxygenated water. Environmental Science & Technology, 46(23), 12913–12920.
Gu, C., Jia, H., Li, H., Teppen, B. J., & Boyd, S. A. (2015). Synthesis of highly reactive subnano-sized zero-valent iron using smectite clay templates. Environmental Science & Technology, 44(11), 4258–4263.
He, F., & Zhao, D. (2007). Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers. Environmental Science & Technology, 41(17), 6216.
Jeong, H. Y., Han, Y. S., Park, S. W., & Hayes, K. F. (2010). Aerobic oxidation of mackinawite (FeS) and its environmental implication for arsenic mobilization. Geochimica et Cosmochimica Acta, 74(11), 3182–3198.
Jiao, C., Cheng, Y., Fan, W., & Li, J. (2015). Synthesis of agar-stabilized nanoscale zero-valent iron particles and removal study of hexavalent chromium. International journal of Environmental Science and Technology, 12(5), 1603–1612.
Kim, E. J., Kim, J. H., Azad, A. M., & Chang, Y. S. (2011). Facile synthesis and characterization of Fe/FeS nanoparticles for environmental applications. ACS Applied Materials & Interfaces, 3(5), 1457–1462.
Lee, Y., Estevez, R., & Kim, C. (2017). Application of commercial coffee for the remediation of hexavalent chromium-contaminated water. Water, Air, and Soil Pollution, 228(4), 161.
Lin, Y. H., Tseng, H. H., Wey, M. Y., & Lin, M. D. (2010). Characteristics of two types of stabilized nano zero-valent iron and transport in porous media. The Science of the Total Environment, 408(10), 2260–2267.
Manning, B. A., Kiser, J. R., Hancheol, K., & Sushil Raj, K. (2007). Spectroscopic investigation of Cr(III)- and Cr(VI)-treated nanoscale zerovalent iron. Environmental Science & Technology, 41(2), 586.
Montesinos, V. N., Quici, N., Halac, E. B., Leyva, A. G., Custo, G., Bengio, S., et al. (2014). Highly efficient removal of Cr(VI) from water with nanoparticulated zerovalent iron: Understanding the Fe(III)–Cr(III) passive outer layer structure. Chemical Engineering Journal, 244(1), 569–575.
Mueller, N. C., Braun, J., Bruns, J., Rissing, M. Č., Rickerby, P. D., et al. (2012). Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environmental Science and Pollution Research International, 19(2), 550–558.
Nurmi, J. T., Tratnyek, P. G., Sarathy, V., Baer, D. R., Amonette, J. E., Pecher, K., et al. (2005). Characterization and properties of metallic iron nanoparticles: Spectroscopy, electrochemistry, and kinetics. Environmental Science & Technology, 39(5), 1221–1230.
O’Carroll, D., Sleep, B., Krol, M., Boparai, H., & Kocur, C. (2013). Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Advances in Water Resources, 51(1), 104–122.
Patterson, R. R., & Fendorf, S. (1997). Reduction of hexavalent chromium by amorphous iron sulfide. Environmental Science & Technology, 31(7), 2039–2044.
Rashmi, S. H., Madhu, G. M., Kittura, A. A., & Suresh, R. (2013). Synthesis, characterization and application of zero valent iron nanoparticles for the removal of toxic metal hexavalent chromium [Cr(VI)] from aqueous solution. International Journal of Current Engineering and Technology, 1, 37–42.
Recillas, S., García, A., González, E., Casals, E., Puntes, V., Sánchez, A., et al. (2011). Use of CeO2, TiO2 and Fe3O4 nanoparticles for the removal of lead from water: Toxicity of nanoparticles and derived compounds. Desalination, 277(1), 213–220.
Reyhanitabar, A., Alidokht, L., Khataee, A. R., & Oustan, S. (2012). Application of stabilized Fe0 nanoparticles for remediation of Cr(VI)-spiked soil. European Journal of Soil Science, 63(5), 724–732.
Rongbing, F., Yingpin, Y., Zhen, X., Xian, Z., Xiaopin, G., & Dongsu, B. (2015). The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere, 138, 726–734.
Saberinasr, A., Rezaei, M., Nakhaei, M., & Hosseini, S. M. (2016). Transport of CMC-stabilized nZVI in saturated sand column: The effect of particle concentration and soil grain size. Water, Air, and Soil Pollution, 227(10), 394.
Singh, J. & Kalamdhad, A. S. (2011). Effects of heavy metals on soil, plants, human health and aquatic life. 1(2), 15–21
Singh, R., Misra, V., & Singh, R. P. (2011). Synthesis, characterization and role of zero-valent iron nanoparticle in removal of hexavalent chromium from chromium-spiked soil. Journal of Nanoparticle Research, 13(9), 4063–4073.
Su, C., Puls, R. W., Krug, T. A., Watling, M. T., O’Hara, S. K., Quinn, J. W., et al. (2012). A two and half-year-performance evaluation of a field test on treatment of source zone tetrachloroethene and its chlorinated daughter products using emulsified zero valent iron nanoparticles. Water Research, 46(16), 5071–5084.
Su, Y., Adeleye, A. S., Keller, A. A., Huang, Y., Dai, C., Zhou, X., et al. (2015). Magnetic sulfide-modified nanoscale zerovalent iron (S-nZVI) for dissolved metal ion removal. Water Research, 74, 47–57.
Su, Y., Adeleye, A. S., Huang, Y., Zhou, X., Keller, A. A., & Zhang, Y. (2016). Direct synthesis of novel and reactive sulfide-modified nano iron through nanoparticle seeding for improved cadmium-contaminated water treatment. Scientific Reports, 6, 24358.
Sun, Y., Li, J., Huang, T., & Guan, X. (2016). The influences of iron characteristics, operating conditions and solution chemistry on contaminants removal by zero-valent iron: A review. Water Research, 100, 277–295.
Tosco, T., Papini, M. P., Viggi, C. C., & Sethi, R. (2014). Nanoscale zerovalent iron particles for groundwater remediation: A review. Journal of Cleaner Production, 77, 10–21.
Xu, Y., & Schoonen, M. A. A. (2000). The absolute energy position of conduction and valence bands of selected semiconducting minerals. American Mineralogist, 85(4), 543–556.
Yamashita, T., & Hayes, P. (2008). Analysis of XPS spectra of Fe 2+ and Fe 3+ ions in oxide materials. Applied Surface Science, 254(8), 2441–2449.
Zhao, X., Liu, W., Cai, Z., Han, B., Qian, T., & Zhao, D. (2016). An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Research, 100, 245–266.
Acknowledgments
We thank the reviewers of this manuscript for their invaluable comments.
Funding
This study was supported financially by the National Natural Science Foundation of China (No. 21607052).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, L., Zhao, Y., Yang, B. et al. Application of Carboxymethyl Cellulose–Stabilized Sulfidated Nano Zerovalent Iron for Removal of Cr(VI) in Simulated Groundwater. Water Air Soil Pollut 230, 113 (2019). https://doi.org/10.1007/s11270-019-4166-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4166-1