Abstract
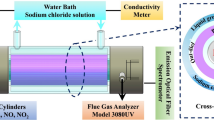
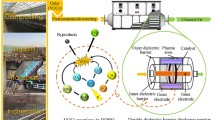
NO is a deadly pollutant that makes up more than 90% NOx in a flue gas. NO is insoluble in water; thus, its removal is extremely difficult using the SCR method. This study investigates the feasibility of NO removal and energy efficiency from a coal-combustion flue gas connected to a DBD reactor merged with urea. The results showed that the combined effect of urea and the DBD reactor gave a significant increase in the removal of NO. Comparing the removal efficiencies of NO at different urea mass percentages (9%, 17%, 23%, 29%, and 33%), it was found that the optimum concentration of urea for efficient NO removal was 23% because there is enough NH3 produced at this concentration and less H2O species generated to consume N• free radicals by OH to produce more NO in the system. The removal efficiencies of the optimum 23% urea concentration with oxygen [(NO/N2/O2/CO(NH2)2:23% w/w system] and without oxygen [(NO/N2/CO(NH2)2:23% w/w system] were also compared. It was also found that the addition of urea solution showed a considerably good energy efficiency which implies that the cost of this technology will be effective. The study complements past studies on the efficient removal of NO from the environment.







Similar content being viewed by others
References
Abdullah, J. A. (2016). Fundamental and Applied Studies of Non-thermal Plasma. University of Newcastle upon Tyne. School of Chemical Engineering and Advanced Materials, Publisher Newcastle University, (196 pages).
Act, C.S., Agency, T., Act, C.S., & Web, C.S. (2002). Overview of the human health and environmental effects of power generation: Focus on sulfur dioxide (SO 2 ), nitrogen oxides (NO X ) and mercury (Hg).
Agency., U. S. E. P (1999). Nitrogen oxides (NOx), why and how they are controlled, (November).
Barbooti, M. M., Ibraheem, N. K., & Ankosh, A. H. (2011). Removal of nitrogen dioxide and sulfur dioxide from air streams by absorption in urea solution. Journal of Environmental Protection., 2011(April), 175–185 https://doi.org/10.4236/jep.2011.22020.
Burtraw, D., Evans, D. A., Krupnick, A., Palmer, K., Toth, R., Burtraw, D., … Toth, R. (2005). Economics of pollution trading for SO2 and NOx, (x).
Finlayson, B.A., Finlayson, B.A., & Engineering, C. (2016). Ullmann ’ s encyclopedia of industrial chemistry. https://doi.org/10.1002/14356007.b01.
Hatton, J., Peter Bulionis, P.E. (2008). A case study of the selective catalytic Reduction (SCR) system at the Algonquin Power Energy-From-Waste Facility (pp. 3–8). Philadelphia, Pennsylvania, USA.
Hu, X., Nicholas, J., Zhang, J. J., de Linjewile, T. M., Filippis, P., & Agarwal, P. K. (2002a). The destruction of N2O in a pulsed corona discharge reactor. Fuel, 81, 1259–1268.
Hu, X., Nicholas, J., Zhang, J., Linjewile, T.M., De Filippis, P., & Agarwal, P K. (2002b). The destruction of N2O in a pulsed corona discharge reactor q. Fuel, 81.
Hueso, J. L., Gonzalez-Elipe, A. R., Cotrino, J., & Caballero, A. (2007). Removal of NO in NO/N2 , NO/N2/O2, NO/CH4/N2, and NO/CH4/O2/N2 systems by flowing microwave discharges. The Journal of Physical Chemistry A, 111, 1057–1065.
Kim, H. (2004). Nonthermal plasma processing for air-pollution control: A historical review. Current Issues, and Future Prospects, 91–110. https://doi.org/10.1002/ppap.200400028.
Koebel, M., Elsener, M., & Kleemann, M. (2000). Urea-SCR: A promising technique to reduce NO x emissions from automotive diesel engines. 59(x):335–345.
Kudo, Y., Taguchi, H., & Kogoshi, S. (2008). Influence of ratio of NO/NO2 on NOx removal using DBD with urea solution. In 11th International Conference on Electrostatic Precipitation, Hangzhou (pp. 1–4).
Leipold, F., Fateev, A., Kusano, Y., Stenum, B., & Bindslev, H. (2006). Reduction of NO in the exhaust gas by reaction with N radicals. Fuel, 85(2), 1383–1388. https://doi.org/10.1016/j.fuel.2005.12.020.
Levine, J. S., Augustsson, T. R., Anderson, I. C., & Hoell, J. M., Jr. (1984). Tropospheric sources of NOx: Lightning and biology. Atmospheric Environment, 18(9), 1797–1804. https://doi.org/10.1016/0004-6981(84)90355-X.
Maiella, P. G., & Brill, T. B. (1996). Spectroscopy of hydrothermal reactions III: The water-gas reaction , “hot spots”, and formation of volatile salts 720 K and 276 bar by T-Jump/FT-IR spectroscopy. Society for Applied Spectroscopy, 50(7), 829–835.
Matsumoto, T., Wang, D., Namihira, T., & Akiyama, H. (2012). Non-thermal plasma technic for air pollution control.
Najjar, Y. S. H. (2011). Gaseous pollutants formation and their harmful effects on health and environment. 1, 1–8. https://doi.org/10.4303/iep/E101203
National Bureau of Statistics of China (2014). Statistical Communiqué of the People’s Republic of China on the 2013. National Economic and Social Development. Retrieved from http://www.stats.gov.cn/english/PressRelease/201402/t20140224_515103.html.
Olivier, J.G.J., Janssens-Maenhout, G., Muntean, M., Peters, J.A.H.W. (2015). Trends in global CO2 emissions 2015. Agency, PBL Netherlands Environmental Assessment. Retrieved from http://edgar.jrc.ec.europa.eu/news_docs/jrc-2015-trends-in-global-co2-emissions-2015-report-98184.pdf.
Omidvarborna, H., Kumar, A., & Kim, D. (2015). NOx emissions from low-temperature combustion of biodiesel made of various feedstocks and blends. 140(x), 113–115.
Penetrante, B. M., Brusasco, R. M., Merritt, B. T., Pitz, W. J., Vogtlin, G. E., & Nal, N. T. F. R. N. A. T. I. O. (1998). Plasma-assisted catalytic reduction of NO. Society for Automotive Engineering, (724).
Ping, F., Chao-ping, C., Xin-ming, W., Zi-jun, T., Zhi-xiong, T., & Ding-sheng, C. (2013). Simultaneous removal of SO2 , NO and Hg 0 by wet scrubbing using urea + KMnO 4 solution. Fuel Processing Technology, 106, 645–653. https://doi.org/10.1016/j.fuproc.2012.09.060.
Prieto, G., Takashima, K., Mizuno, A., Prieto, O., & Gay, C. R. (2013). Dielectric barrier discharge for ammonia production. Plasma Chemistry and Plasma Processing, 337–353. https://doi.org/10.1007/s11090-012-9428-2.
Reduction, S. C., Device, C., Pollutants, A., Oxides, N., Limits, A. E., Type, A. S., … Considerations, O. (2002). Air Pollution Control Technology Fact Sheet, 1–5.
Revkin A.C. (2015). A look behind the headlines on China’s coal trends. The New York Times. Retrieved from http://dotearth.blogs.nytimes.com/2015/02/18/a-look-behind-the-headlines-on-chinas-coal-trends/?_r=1.
Sakamaki, T., Murata, H., Kogoshi, S. (2006). NOx removal using DBD with urea solution and plasma treated TiO2 photo-catalyst. In ICESP X-Auatralia 2006 (pp. 1–7).
Schaber, P. M., Colson, J., Higgins, S., Thielen, D., Anspach, B., & Brauer, J. (2004). Thermal decomposition ( pyrolysis ) of urea in an open reaction vessel. Thermochimica Acta, 424, 131–142. https://doi.org/10.1016/j.tca.2004.05.018.
Shui-E, Y., Bao-Min, S., Xu-Dong, G., Hai-Ping, Xiao. (2009). The Effect of Oxygen and Water Vapor on Nitric Oxide Conversion with a Dielectric Barrier Discharge Reactor. Plasma Chem Plasma Process, 29(2), 421–31. https://doi.org/10.1007/s11090-009-9190-2.
States, U. (1998). NOx What is it? Where does it come from ?, (September).
Stradella, L., & Argentero, M. (1993). A study of the thermal decomposition of urea , of related compounds and thiourea using DSC and TG-EGA. Thermochimica Acta, 219, 315–323.
Wang, J., Wu, C., Chen, J., & Zhang, H. (2006). Denitrification removal of nitric oxide in a rotating drum biofilter. 121(x), 45–49. https://doi.org/10.1016/j.cej.2006.04.004.
Wei, J., Luo, Y., Yu, P., Cai, B., & Tan, H. (2009). Journal of industrial and engineering chemistry removal of NO from flue gas by wet scrubbing with NaClO2/(NH2)2CO solutions. 15, 2008–2010. https://doi.org/10.1016/j.jiec.2008.07.010.
Xiao, X.C., X. N. W. (2005). Study on pollution and control of NOX in air environment. Coal Technol, (24), 1–2.
Yearbook, S. (2012). Statistical yearbook.
Yeates, G.L., & Nielsen, D.M.. (2000). The effects of acid precipitation on ground water quality in the northeastern United States.
Zhao, B., Wang, S. X., Liu, H., Xu, J. Y., Fu, K., Klimont, Z., … Amann, M. (2013). NOx emissions in China: Historical trends and future perspectives. 9869–9897. https://doi.org/10.5194/acp-13-9869-2013
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jingyu, H., Opoku, P.A., Junwei, L. et al. Two-Staged Dielectric Barrier Discharge-Urea Configuration for the Removal of NO of NOx in a Simulated Coal-Combustion Flue Gas. Water Air Soil Pollut 230, 120 (2019). https://doi.org/10.1007/s11270-019-4165-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4165-2