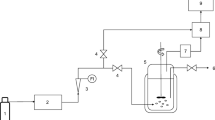
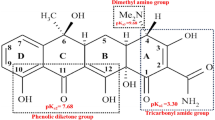
Abstract
Huge amounts of natural bischofite (MgCl2∙6H2O) resulting from the mining process of salt lakes often cannot be utilized effectively and are discarded; techniques for reutilization of the discarded bischofite as magnesium resources are limited. The nano-magnesium hydroxide (nano-Mg(OH)2) synthesized from natural bischofite was firstly used as catalyst for ozonation of antibiotics including sulfathiazole (ST), ofloxacin (OFL), and tetracycline (TC). Rapid ozonation of ST, OFL, and TC was achieved using nano-Mg(OH)2 as catalyst. The removal rate constant of OFL in the catalytic ozonation treatment (kOFL = 0.512 min−1) was nearly 2.1 times higher than the single ozonation (kOFL = 0.249 min−1). The removal rate constant of ST and TC increased by 23.5% and 32.8% from 0.298 min−1 and 0.384 min−1 to 0.368 min−1 and 0.510 min−1, respectively, when the catalyst was added into the reaction system. The removal rate constant of ST sharply increased from 0.259 to 0.604 min−1 when the reaction temperature increased from 15 to 35 °C while those of OFL and TC changes slightly. The removal efficiency sharply decreased when the initial concentration of ST, OFL, and TC increased from 10 to 500 mg L−1. Both anions and cations could inhibit the removal of ST, OFL, and TC at relatively higher concentrations. The prepared nano-Mg(OH)2 catalyst could maintain its catalytic activity in the repeated use process. High removal efficiency of typical antibiotics and heavy metals free indicated that nano-Mg(OH)2 from natural bischofite is a promising ozonation catalyst in terms of antibiotics removal.









Similar content being viewed by others
References
Amutha, R., Sillanpää, M., Lee, G. J., Lin, J. C., Yang, C. K., & Wu, J. J. (2014). Catalytic ozonation of 2-ethoxy ethyl acetate using mesoporous nickel oxalates. Catalysis Communications, 43, 88–92.
Bai, Z., Yang, Q., & Wang, J. (2016). Catalytic ozonation of sulfamethazine using Ce0.1Fe0.9OOH as catalyst: Mineralization and catalytic mechanisms. Chemical Engineering Journal, 300, 169–176.
Brown, K. D., Kulis, J., Thomson, B., Chapman, T. H., & Mawhinney, D. B. (2006). Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Science of the Total Environment, 366, 772–783.
Buxton, G. V. (1988). Critical view of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. Journal of Physical & Chemical Reference Data, 17, 513–886.
Carvalho, I. T., & Santos, L. (2016). Antibiotics in the aquatic environments: A review of the European scenario. Environment International, 94, 736–757.
Changotra, R., Guin, J. P., Varshney, L., & Dhir, A. (2018). Assessment of reaction intermediates of gamma radiation-induced degradation of ofloxacin in aqueous solution. Chemosphere, 208, 606–613.
Dong, Y., He, K., Yin, L., & Zhang, A. (2007). Catalytic degradation of nitrobenzene and aniline in presence of ozone by magnesia from natural mineral. Catalysis Letters, 119, 222–227.
Flamm, D. L. (1977). Analysis of ozone at low concentrations with boric acid buffered KI. Environmental Science & Technology, 11, 978–983.
Giri, R. R., Ozaki, H., Taniguchi, S., & Takanami, R. (2008). Photocatalytic ozonation of 2,4-dichlorophenoxyacetic acid in water with a new TiO2 fiber. International Journal of Environmental Science & Technology, 5, 17–26.
Gonçalves, A. G., Órfão, J. J. M., & Pereira, M. F. R. (2012). Catalytic ozonation of sulphamethoxazole in the presence of carbon materials: Catalytic performance and reaction pathways. Journal of Hazardous Materials, 239-240, 167–174.
He, K., Dong, Y. M., Li, Z., Yin, L., Zhang, A. M., & Zheng, Y. C. (2008). Catalytic ozonation of phenol in water with natural brucite and magnesia. Journal of Hazardous Materials, 159, 587–592.
Hoigné, J., & Bader, H. (1983). Rate constants of reactions of ozone with organic and inorganic compounds in water-I non-dissociating organic compounds. Water Research, 17, 173–183.
Hou, L., Zhang, H., Wang, L., Chen, L., Xiong, Y., & Xue, X. (2013a). Removal of sulfamethoxazole from aqueous solution by sono-ozonation in the presence of a magnetic catalyst. Separation & Purification Technology, 117, 46–52.
Hou, L., Zhang, H., Wang, L., & Chen, L. (2013b). Ultrasound-enhanced magnetite catalytic ozonation of tetracycline in water. Chemical Engineering Journal, 229, 577–584.
Kim, S. D., Cho, J., Kim, I. S., Vanderford, B. J., & Snyder, S. A. (2007). Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Research, 41, 1013–1021.
Kümmerer, K. (2001). Drugs in the environment: Emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources-a review. Chemosphere, 45, 957–969.
Kümmerer, K. (2009). Antibiotics in the aquatic environment—a review—part II. Chemosphere, 75, 435–441.
Kwan, C. Y., & Chu, W. (2004). A study of the reaction mechanisms of the degradation of 2,4-dichlorophenoxyacetic acid by oxalate-mediated photooxidation. Water Research, 38, 4213–4221.
Li, L., Ye, W., Zhang, Q., Sun, F., Ping, L., & Li, X. (2009). Catalytic ozonation of dimethyl phthalate over cerium supported on activated carbon. Journal of Hazardous Materials, 170, 411–416.
Lü, X., Zhang, Q., Yang, W., Li, X., Zeng, L., & Li, L. (2015). Catalytic ozonation of 2,4-dichlorophenoxyacetic acid over novel Fe-Ni/AC. RSC Advances, 5, 10537–10545.
Managaki, S., Murata, A., Takada, H., Tuyen, B. C., & Chiem, N. H. (2007). Distribution of macrolides, sulfonamides, and trimethoprim in tropical waters: Ubiquitous occurrence of veterinary antibiotics in the Mekong Delta. Environmental Science & Technology, 41, 8004–8010.
Miao, X. S., Bishay, F., Chen, M., & Metcalfe, C. D. (2004). Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environmental Science & Technology, 38, 3533–3541.
Moussavi, G., Khavanin, A., & Alizadeh, R. (2010). The integration of ozonation catalyzed with MgO nanocrystals and the biodegradation for the removal of phenol from saline wastewater. Applied Catalysis B Environmental, 97, 160–167.
Peng, X., Wang, Z., Kuang, W., Tan, J., & Li, K. (2006). A preliminary study on the occurrence and behavior of sulfonamides, ofloxacin and chloramphenicol antimicrobials in wastewaters of two sewage treatment plants in Guangzhou, China. Science of the Total Environment, 371, 314–322.
Snyder, S. A., Wert, E. C., Rexing, D. J., Zegers, R. E., & Drury, D. D. (2006). Ozone oxidation of endocrine disruptors and pharmaceuticals in surface water and wastewater. Ozone Science & Engineering, 28, 445–460.
Song, C., Wang, L., Ren, J., Lv, B., Sun, Z., Yan, J., Li, X., & Liu, J. (2016). Comparative study of diethyl phthalate degradation by UV/H2O2 and UV/TiO2: Kinetics, mechanism, and effects of operational parameters. Environmental Science & Pollution Research, 23, 2640–2650.
Tian, Y., Gao, B., Morales, V. L., Chen, H., Wang, Y., & Li, H. (2013). Removal of sulfamethoxazole and sulfapyridine by carbon nanotubes in fixed-bed columns. Chemosphere, 90, 2597–2605.
Trovó, A. G., Nogueira, R. F., Agüera, A., Fernandezalba, A. R., Sirtori, C., & Malato, S. (2009). Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation. Water Research, 43, 3922–3931.
Vieno, N. M., Härkki, H., Tuhkanen, T., & Kronberg, L. (2007). Occurrence of pharmaceuticals in river water and their elimination in a pilot-scale drinking water treatment plant. Environmental Science & Technology, 41, 5077–5084.
Wang, J. L., & Xu, L. J. (2012). Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Critical Reviews in Environmental Science & Technology, 42, 251–325.
Wang, W., Qiao, X., Chen, J., & Li, H. (2007). Facile synthesis of magnesium oxide nanoplates via chemical precipitation. Materials Letters, 61, 3218–3220.
Wang, Y., Zhang, H., Chen, L., Wang, S., & Zhang, D. (2012). Ozonation combined with ultrasound for the degradation of tetracycline in a rectangular air-lift reactor. Separation & Purification Technology, 84, 138–146.
Yan, C., Yang, Y., Zhou, J., Liu, M., Nie, M., Shi, H., & Gu, L. (2013). Antibiotics in the surface water of the Yangtze Estuary: Occurrence, distribution and risk assessment. Environmental Pollution, 175, 22–29.
Yin, R., Guo, W., Du, J., Zhou, X., Zheng, H., Wu, Q., Chang, J., & Ren, N. (2017). Heteroatoms doped graphene for catalytic ozonation of sulfamethoxazole by metal-free catalysis: Performances and mechanisms. Chemical Engineering Journal, 317, 632–639.
Yuan, R., Zhou, B., Hua, D., & Shi, C. (2013). Enhanced photocatalytic degradation of humic acids using Al and Fe co-doped TiO2 nanotubes under UV/ozonation for drinking water purification. Journal of Hazardous Materials, 262, 527–538.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 41671319), One Hundred Talents Program of Chinese Academy of Sciences (grant numbers Y629041021 and Y610061033), Taishan Scholar Program of Shandong Province, Thousand Talents Plan of Qinghai Province (Y740171071), and Two-Hundred Talents Plan of Yantai (No. Y739011021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Q., Lu, J., Wu, J. et al. Catalytic Ozonation of Sulfonamide, Fluoroquinolone, and Tetracycline Antibiotics Using Nano-Magnesium Hydroxide from Natural Bischofite. Water Air Soil Pollut 230, 55 (2019). https://doi.org/10.1007/s11270-019-4108-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4108-y