Abstract
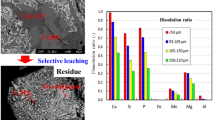
Removal of phosphate using ettringite synthesized from industrial by-products was evaluated in this study. Ettringite was synthesized by combining basic oxygen furnace (BOF) slag, alum, and flue-gas-desulfurization (FGD) gypsum at molar ratio ([Ca]:[Al]:[SO4]) of 3:2:3, pH 11.74, and 28 °C for 24 h. Kinetic study showed that the adsorption of phosphate by ettringite reached equilibrium after 24 h and could be represented by pseudo-second-order kinetic model. Equilibrium adsorption study revealed that pH was the most important factor, and removal efficiency increased with increasing pH. The mode of phosphate removal could be divided into two regions. At lower initial phosphate concentration (< 300 mg/L), experimental results fitted well with both Langmuir and Freundlich isotherm models. However, the adsorption density increased linearly with increasing phosphate concentration when at higher initial phosphate concentration (> 300 mg/L), implying that phosphate was mainly removed by surface precipitation. Judging from X-ray diffraction (XRD) analysis and PHREEQC simulation, the main calcium phosphate precipitate is hydroxyapatite (HAP). This study demonstrated that it is feasible to synthesize highly effective adsorption material using industrial by-products for phosphate removal.









Similar content being viewed by others
References
Alvarez-Ayuso, E., & Nugteren, H. W. (2005). Synthesis of ettringite: a way to deal with the acid wastewaters of aluminium anodising industry. Water Research, 39, 65–72.
Barca, C., Gerente, C., Meyer, D., Chazarenc, F., & Andrer, Y. (2012). Phosphate removal from synthetic and real wastewater using steel slags produced in Europe. Water Research, 46, 2376–2384.
Baur, I., & Johnson, C. A. C.A.(2003). The solubility of selenate-Aft (3CaO·Al2O3·3CaSeO4·37.5H2O) and selenate-AFm (3CaO·Al2O3·CaSeO4·xH2O). Cement and Concrete Research, 33(2003), 1741–1748.
Benyoucef, S., & Amrani, M. (2011). Adsorption of phosphate ions onto low cost Aleppo pine adsorbent. Desalination, 275, 231–236.
Blanco, I., Molle, P., Saenz de Miera, L. E., & Ansola, G. (2016). Basic oxygen furnace steel slag aggregates for phosphorus treatment. Evaluation of its potential use as a substrate in constructed wetlands. Water Research, 89, 355–365.
Chang, E. E., Chiu, A. C., Pan, S. Y., Chen, Y. H., Tan, C. S., & Chiang, P. C. (2013). Carbonation of basic oxygen furnace slag with metalworking wastewater in a slurry reactor. International Journal of Greenhouse Gas Control, 12, 382–389.
Chen, H., Wang, D., Li, X., Yang, Q., Luo, K., & Zeng, G. (2013). Biological phosphorus removal from real wastewater in a sequencing batch reactor operated as aerobic/extended-idle regime. Biochemical Engineering Journal, 77, 147–153.
Collier, N. C., Milestone, N. B., Gordon, L. E., & Ko, S. C. (2014). The suitability of a supersulfated cement for nuclear waste immobilisation. Journal of Nuclear Materials, 452, 457–464.
Gougar, M. L. D., Scheetz, B. E., & Roy, D. M. (1996). Ettringite and C-S-H Portland cement phases for waste ion immobilization: a review. Waste Management, 16, 295–303.
Guan, B., Kong, B., Fu, H., Yu, J., Jiang, G., & Yang, L. (2012). Pilot scale preparation of α-calcium sulfate hemihydrate from FGD gypsum in Ca–K–Mg aqueous solution under atmospheric pressure. Fuel, 98, 48–54.
Gunawan, E. K., Warmadewanthi, & Liu, J. C. (2010). Removal of phosphate and fluoride from optoelectronic wastewater by calcite. International Journal of Environmental Technology and Management, 12, 308–321.
Han, C., Wang, Z., Yang, W., Wu, Q., Yang, H., & Xue, X. (2016). Effects of pH on phosphorus removal capacities of basic oxygen furnace slag. Ecological Engineering, 89, 1–6.
Hashem, F. S., & Amin, M. S. (2014). Kinetic and thermal studies of removal of CrO4 2- ions by ettringite. Journal of Thermal Analysis and Calorimetry, 116, 835–844.
Hermassi, M., Valderrama, C., Dosta, J., Cortina, J. L., & Batis, N. H. (2015). Evaluation of hydroxyapatite crystallization in a batch reactor for the valorization of alkaline phosphate concentrates from wastewater treatment plants using calcium chloride. Chemical Engineering Journal, 267, 142–152.
Hongo, T., Tsunashima, Y., Iizuka, A., & Yamasaki, A. (2014). Synthesis of anion-exchange materials from concrete sludge and evaluation of their ability to remove harmful anions (borate, fluoride, and chromate). International Journal of Chemical Engineering and Applications, 5, 298–302.
Iizuka, A., Takahashi, M., Nakamura, T., & Yamasaki, A. (2014). Boron removal performance of a solid sorbent derived from waste concrete. Industrial and Engineering Chemistry Research, 53, 4046–4051.
Kanchana, P., & Sekar, C. (2010). Influence of sodium fluoride on the synthesis of hydroxyapatite by gel method. Journal of Crystal Growth, 312, 808–816.
Komatsu, R., Mizukoshi, N., Makida, K., & Tsukamoto, K. (2009). In-situ observation of ettringite crystals. Journal of Crystal Growth, 311, 1005–1008.
Köse, T. E., & Kivanc, B. (2011). Adsorption of phosphate from aqueous solutions using calcined waste eggshell. Chemical Engineering Journal, 178, 34–39.
Lu, N. C., & Liu, J. C. (2010). Removal of phosphate and fluoride from wastewater by a hybrid precipitation–microfiltration process. Separation and Purification Technology, 74, 329–335.
Madzivire, G., Petrik, L. F., Gitari, W. M., Ojumu, T. V., & Balfour, G. (2010). Application of coal fly ash to circumneutral mine waters for the removal of sulphates as gypsum and ettringite. Mining and Engineering, 23, 252–257.
Myneni, S. C. B., Traina, S. J., Logan, T. J., & Waychunas, G. A. (1997). Oxyanion behavior in alkaline environments: sorption and desorption of arsenate in ettringite. Environmental Science & Technology, 31, 1761–1768.
Novillo, C., Guaya, D., Avendaño, A. A.-P., Armijos, C., Cortina, J. L., & Cota, I. (2014). Evaluation of phosphate removal capacity of Mg/Al layered double hydroxides from aqueous solutions. Fuel, 38, 72–79.
Parkhurst, D. L., & Appelo, C. A. J. (1999). User’s guide to PHREEQC (version 2)—a computer program, for speciation, batch reaction, one-dimensional transport, and inverse geochemical calculations. Denver: U. S. Geological Survey Water-Resource Investigations Report 99–4259, USGS.
Perassi, I., & Borgnino, L. (2014). Adsorption and surface precipitation of phosphate onto CaCO3–montmorillonite: effect of pH, ionic strength and competition with humic acid. Geoderma, 232-234, 600–608.
Qiu, L., Zheng, P., Zhang, M., Yu, X., & Chulam, A. (2015). Phosphorus removal using ferric-calcium complex as precipitant: parameters optimization and phosphorus-recycling potential. Chemical Engineering Journal, 268, 230–235.
Rathod, M., Mody, K., & Basha, S. (2014). Efficient removal of phosphate from aqueous solutions by red seaweed, Kappaphycus alverezii. Journal of Cleaner Production, 84, 484–493.
Saikia, N., Kato, S., & Kojima, T. (2006). Behavior of B, Cr, Se, As, Pb, Cd, and Mo present in waste leachates generated from combustion residues during the formation of ettringite. Environmental Toxicology and Chemistry, 25, 1710–1719.
Sasaki, T., Lizuka, A., Watanabe, M., Hongo, T., & Yamasaki, A. (2014). Preparation and performance of arsenate (V) adsorbents derived from concrete wastes. Waste Management, 34, 1829–1835.
Soetardji, J. P., Claudia, J. C., Ju, Y. H., Hriljac, J. A., Chen, T. Y., Soetaredjo, F. E., Santoso, S. P., Kurniawan, A., & Ismadji, S. (2015). Ammonia removal from water using sodium hydroxide modified zeolite mordenite. RSC Advances, 5, 83689–83699.
Tsunashima, Y., Iizuka, A., Akimoto, J., Hongo, T., & Yamasaki, A. (2012). Preparation of sorbents containing ettringite phase from concrete sludge and their performance in removing borate and fluoride ions from waste water. Chemical Engineering Journal, 200–202, 338–343.
Wajima, T., & Rakovan, J. F. (2013). Removal behavior of phosphate from aqueous solution by calcined paper sludge. Colloid Surfaces A: Physicochemical and Engineering Aspects, 432, 132–138.
Wang, T., Dorner-Reisel, A., & Ller, E. M. (2004). Thermogravimetric and thermokinetic investigation of the dehydroxylation of a hydroxyapatite powder. Journal of the European Ceramic Society, 24, 693–698.
Xu, K., Deng, T., Liu, J., & Peng, W. (2010). Study on the phosphate removal from aqueous solution using modified fly ash. Fuel, 89, 3668–3674.
Yagi, S., & Fukushi, F. (2012). Removal of phosphate from solution by adsorption and precipitation of calcium phosphate onto monohydrocalcite. Journal of Colloid and Interface Science, 384, 128–136.
Yan, Y., Sun, X., Ma, F., Li, J., Shen, J., Han, W., Liu, X., & Wang, L. (2014). Removal of phosphate from wastewater using alkaline residue. Journal of Environmental Sciences, 26, 970–980.
Yang, S., Jin, P., Wang, X., Zhang, Q., & Chen, X. (2016). Phosphate recovery through adsorption assisted precipitation using novel precipitation material developed from building waste: behavior and mechanism. Chemical Engineering Journal, 292, 246–254.
Yu, Y., Wu, R., & Clark, M. (2010). Phosphate removal by hydrothermally modified fumed silica and pulverized oyster shell. Journal of Colloid and Interface Science, 350, 538–543.
Zhang, M., & Reardon, E. J. (2003). Removal of B, Cr, Mo, and Se from wastewater by incorporation into hydrocalumite and ettringite. Environmental Science & Technology, 37, 2947–2952.
Zhou, W., Huang, Z., Sun, C., Zhao, H., & Zhang, Y. (2016). Enhanced phosphorus removal from wastewater by growing deep-sea bacterium combined with basic oxygen furnace slag. Bioresource Technology, 214, 534–540.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, RJ., Liu, JC. Removal of Phosphate Using Ettringite Synthesized from Industrial By-products. Water Air Soil Pollut 229, 185 (2018). https://doi.org/10.1007/s11270-018-3828-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3828-8