Abstract
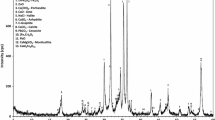
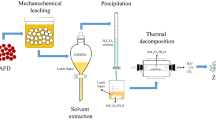
Electric arc furnace dust (EAFD) is a toxic waste which is mainly rich in iron oxide, zinc, and lead. Hydrometallurgical extraction of zinc from Jordanian EAFD in alkaline medium was investigated; NaOH, NaHCO3, and Na2CO3 were used as leaching agents. The pH values for the prepared solutions were 8.3, 8.2, and 12.55 for NaHCO3, Na2CO3, and NaOH, respectively. The effect of NaOH concentration (1, 3, 5, 7, and 9 M), contact time (5 min to 3 h), temperature (20, 40, and 60), and solid-to-liquid ratio (SLR; 20, 40, 80, and 120 mg/ml) on the leachability of zinc from EAFD were tested. The initial EAFD and the resulting leach residues were characterized using X-ray diffraction (XRD) and X-ray fluorescence (XRF). EAFD contained 25.9% Zn, 18.0% Fe, and 3.2% Pb. A maximum zinc recovery of 92.9% was achieved using 6 M NaOH at 60 °C with solid loading of 20 g/L and 3 h leaching time. NaHCO3 and Na2CO3 were not efficient leaching agents for Zn extraction since the recoveries were only 2.6 and 4.5%, respectively. Zn and Pb were depleted in the residues with an E-factor of 0.5–0.6 and 0.1–0.25, respectively. Iron was enriched in the residues; the E-factor was around 2. The EAFD contained mainly zincite, franklinite, and magnetite. After 3 h leaching, only traces of zincite exist in the residues, while sylvite and halite were completely dissolved.









Similar content being viewed by others
References
Abkhoshk, E., Jorjani, E., Al-Harahsheh, M. S., Rashchi, F., & Naazeri, M. (2014). Review of the hydrometallurgical processing of non-sulfide zinc ores. Hydrometallurgy, 149, 153–167.
Ahmed, O. H., Altarawneh, M., Al-Harahsheh, M., Jiang, Z.-T., & Dlugogorski, B. Z. (2017). Recycling of zincite (ZnO) via uptake of hydrogen halides. Physical Chemistry Chemical Physics.
Al-Harahsheh, M., & Kingman, S. W. (2004). Microwave-assisted leaching—a review. Hydrometallurgy, 73, 189–203.
Al-Harahsheh, M., Al-Otoom, A., Al-Makhadmah, L., Hamilton, I. E., Kingman, S., Al-Asheh, S., & Hararah, M. (2015). Pyrolysis of poly(vinyl chloride) and-electric arc furnacedust mixtures. Journal of Hazardous Materials, 299, 425–436.
de Araujo, J. A., & Schalch, V. (2014). Recycling of electric arc furnace (EAF) dust for use in steel making process. Journal of Materials Research and Technology, 3, 274–279.
Barrett, E. C., Nenniger, E. H., & Dziewinski, J. (1992). A hydrometallurgical process to treat carbon steel electric arc furnace dust. Hydrometallurgy, 30, 59–68.
Caravaca, C., Cobo, A., & Alguacil, F. J. (1994). Considerations about the recycling of EAF flue dusts as source for the recovery of valuable metals by hydrometallurgical processes. Resources, Conservation and Recycling, 10, 35–41.
Dreisinger, D. (1990). A challenge for the 1990s: the hydrometallurgical treatment of wastes and residues. JOM, 42, 27–27.
Dutra, A. J. B., Paiva, P. R. P., & Tavares, L. M. (2006). Alkaline leaching of zinc from electric arc furnace steel dust. Minerals Engineering, 19, 478–485.
Elgersma, F., Kamst, G. F., Witkamp, G. J., & van Rosmalen, G. M. (1992). Acidic dissolution of zinc ferrite. Hydrometallurgy, 29, 173–189.
Frenay, J., Ferlay, S. & Hissel, J.: 1986, Zinc and lead recovery from EAF dusts by caustic soda process. In: Electric furnace proceedings, treatment options for carbon steel electric arc furnace dust, Iron Steel Society, pp. 171–175.
Havlík, T., Vidor e Souza, B., Bernardes, A. M., Schneider, I. A., & Miškufová, A. (2006). Hydrometallurgical processing of carbon steel EAF dust. Journal of Hazardous Materials, 135, 311–318.
Jha, M. K., Kumar, V., & Singh, R. J. (2001). Review of hydrometallurgical recovery of zinc from industrial wastes. Resources, Conservation and Recycling, 33, 1–22.
Kavouras, P., Kehagias, T., Tsilika, I., Kaimakamis, G., Chrissafis, K., Kokkou, S., Papadopoulos, D., & Karakostas, T. (2007). Glass-ceramic materials from electric arc furnace dust. Journal of Hazardous Materials, 139, 424–429.
Kekki, A., Aromaa, J., & Forcen, O. (2012). Leaching characteristics of EAF and AOF stainless steel production dusts. Physicochemical Problems of Mineral Processing, 48, 599–606.
Kelebek, S., Yörük, S., & Davis, B. (2004). Characterization of basic oxygen furnace dust and zinc removal by acid leaching. Minerals Engineering, 17, 285–291.
Leclerc, N., Meux, E., & Lecuire, J.-M. (2003). Hydrometallurgical extraction of zinc from zinc ferrites. Hydrometallurgy, 70, 175–183.
Lenntech, B. V.: 2017, Zinc and water: reaction mechanisms, environmental impact and health effects. Distributieweg 3, EG Delfgauw: Lenntech.
Li, H.-X., Wang, Y., & Cang, D.-Q. (2010). Zinc leaching from electric arc furnace dust in alkaline medium. Journal of Central South University of Technology, 17, 967–971.
Mordogan, H., Cicek, T., & Isik, A. (1999). Caustic soda leach of electric arc furnace dust. J. Eng. Environ. Sci., 23, 199–207.
Nagib, S., & Inoue, K. (2000). Recovery of lead and zinc from fly ash generated from municipal incineration plants by means of acid and/or alkaline leaching. Hydrometallurgy, 56, 269–292.
Niubo, M., Fernandez, A. I., Chimenos, J. M., & Haurie, L. (2009). A possible recycling method for high grade steels EAFD in polymer composites. Journal of Hazardous Materials, 171, 1139–1144.
Oishi, T., Yaguchi, M., Koyama, K., Tanaka, M., & Lee, J. C. (2008). Hydrometallurgical process for the recycling of copper using anodic oxidation of cuprous ammine complexes and flow-through electrolysis. Electrochimica Acta, 53, 2585–2592.
Orhan, G. (2005). Leaching and cementation of heavy metals from electric arc furnace dust in alkaline medium. Hydrometallurgy, 78, 236–245.
Oustadakis, P., Tsakiridis, P. E., Katsiapi, A., & Agatzini-Leonardou, S. (2010). Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). Part I. Characterization and leaching by diluted sulphuric acid. Journal of Hazardous Materials, 179, 1–7.
Sofilić, T., Rastovčan-Mioč, A., Cerjan-Stefanović, Š., Novosel-Radović, V., & Jenko, M. (2004). Characterization of steel mill electric-arc furnace dust. Journal of Hazardous Materials, 109, 59–70.
Stegemann, J. A., Roy, A., Caldwell, R. J., Schilling, P. J., & Tittsworth, R. (2000). Understanding environmental leachability of electric arc furnace dust. Journal of Environmental Engineering, 126, 112–120.
Suetens, T., Klaasen, B., Van Acker, K., & Blanpain, B. (2014). Comparison of electric arc furnace dust treatment technologies using exergy efficiency. Journal of Cleaner Production, 65, 152–167.
Vieira, C. M. F., Sanchez, R., Monteiro, S. N., Lalla, N., & Quaranta, N. (2013). Recycling of electric arc furnace dust into red ceramic. Journal of Materials Research and Technology, 2, 88–92.
Xia, D. K., & Picklesi, C. A. (2000). Microwave caustic leaching of electric arc furnace dust. Minerals Engineering, 13, 79–94.
Youcai, Z., & Stanforth, R. (2000). Integrated hydrometallurgical process for production of zinc from electric arc furnace dust in alkaline medium. Journal of Hazardous Materials, 80, 223–240.
Zhang, Y., Li, X., Pan, L., Wei, Y., & Liang, X. (2010). Effect of mechanical activation on the kinetics of extracting indium from indium-bearing zinc ferrite. Hydrometallurgy, 102, 95–100.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Makhadmeh, L.A., Batiha, M.A., Al-Harahsheh, M.S. et al. The Effectiveness of Zn Leaching from EAFD Using Caustic Soda. Water Air Soil Pollut 229, 33 (2018). https://doi.org/10.1007/s11270-018-3694-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3694-4