Abstract
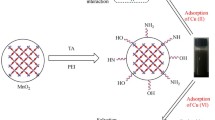
Modified natural biosorbents have been the research hot spot in wastewater treatment field. Natural polyphenol compounds, tannins with many functional groups can be easily modified to produce biosorbents. This work presents an environmentally friendly tannin-based dithiocarbamate (TDTC) with enhanced function of adsorption. TDTC was successfully synthesized by a two-step method involving Mannich reaction and esterification. The structure and synthetic mechanisms of TDTC were discussed through instrumental analysis. The BET-specific surface area analysis results showed that TDTC exhibited a mesoporous structure with an increased surface area. Parameters affecting adsorption such as initial pH, adsorbent dosage, contact time, initial concentration, and temperature were systematically examined. Results showed that the maximum adsorption capacity toward Ni(II) of TDTC was 112.49 mg/g. In addition, TDTC exhibited a fast adsorption rate and 80% of the Ni(II) was removed within just 20 min. The adsorption kinetics and adsorption isotherm were well described by the pseudo-second-order model and Langmuir model, respectively. Moreover, TDTC could be easily regenerated without obvious loss of adsorption capability after five adsorption–desorption cycles. All these results indicate that TDTC is an ideal candidate for the efficient removal of Ni(II).

Removal of Ni(II) from water using environmental-friendly tannin-based dithiocarbamate biosorbents.











Similar content being viewed by others
Abbreviations
- TDTC:
-
tannin-based dithiocarbamate
- FTIR:
-
Fourier transform-infrared spectroscopy
- FSEM:
-
field-emission scanning electron microscopy
- EDS:
-
energy-dispersive spectroscopy
- XPS:
-
X-ray photoelectron spectroscopy
- BT:
-
bayberry tannin
- R:
-
removal efficiency
- Qe :
-
adsorbed amount at equilibrium
References
Akter, N., Hossain, M. A., Hassan, M. J., Amin, M. K., Elias, M., Rahman, M. M., Asiri, A. M., Siddiquey, I. A., & Hasnat, M. A. (2016). Amine modified tannin gel for adsorptive removal of Brilliant Green dye. Journal of Environmental Chemical Engineering, 4(1), 1231–1241.
Bakalar, T., Bugel, M., & Gajdosova, L. (2009). Heavy metal removal using reverse osmosis. Acta Montanistica Slovaca, 14(3), 250–253.
Bediako, J. K., Wei, W., Kim, S., & Yun, Y. (2015). Removal of heavy metals from aqueous phases using chemically modified waste Lyocell fiber. Journal of Hazardous Materials, 299, 550–561.
Beltran Heredia, J., & Sanchez Martin, J. (2009). Removing heavy metals from polluted surface water with a tannin-based flocculant agent. Journal of Hazardous Materials, 165(1–3), 1215–1218.
Beltran-Heredia, J., Sanchez-Martin, J., & Davila-Acedo, M. A. (2011). Optimization of the synthesis of a new coagulant from a tannin extract. Journal of Hazardous Materials, 186(2–3), 1704–1712.
Beltran-Heredia, J., Palo, P., Sanchez-Martin, J., Dominguez, J. R., & Gonzalez, T. (2012). Natural adsorbents derived from tannin extracts for pharmaceutical removal in water. Industrial & Engineering Chemistry Research, 51(1), 50–57.
Chakraborti, R. K., Gardner, K. H., Atkinson, J. F., & Van Benschoten, J. E. (2003). Changes in fractal dimension during aggregation. Water Research, 37(PII S0043–1354(02)00379–24), 873–883.
Deng, S., Wang, P., Zhang, G., & Dou, Y. (2016). Polyacrylonitrile-based fiber modified with thiosemicarbazide by microwave irradiation and its adsorption behavior for Cd(II) and Pb(II). Journal of Hazardous Materials, 307, 64–72.
Dermentzis, K. (2010). Removal of nickel from electroplating rinse waters using electrostatic shielding electrodialysis/electrodeionization. Journal of Hazardous Materials, 173(1–3), 647–652.
Ewecharoen, A., Thiravetyan, P., & Nakbanpote, W. (2008). Comparison of nickel adsorption from electroplating rinse water by coir pith and modified coir pith. Chemical Engineering Journal, 137(2), 181–188.
Freundlich, H. (1906). Concerning adsorption in solutions. Zeitschrift Fur Physikalische Chemie--Stochiometrie Und Verwandtschaftslehre, 57(4), 385–470.
Furlani, C., Polzonetti, G., Preti, C., & Tosi, G. (1983). XPS of coordination compounds: data on the electronic structure of a series of Cu(II) N,N′-cyclic substituted dithiocarbamates. Inorganica Chimica Acta-Articles, 73(1), 105–111.
Ge, Y., Li, Z., Kong, Y., Song, Q., & Wang, K. (2014). Heavy metal ions retention by bi-functionalized lignin: synthesis, applications, and adsorption mechanisms. Journal of Industrial and Engineering Chemistry, 20(6), 4429–4436.
Ge, Y., Xiao, D., Li, Z., & Cui, X. (2015a). Dithiocarbamate functionalized lignin for efficient removal of metallic ions and the usage of the metal-loaded bio-sorbents as potential free radical scavengers (vol 2, pg 2136, 2014). Journal of Materials Chemistry A, 3(14), 7666.
Ge, Y., Song, Q., & Li, Z. (2015b). A Mannich base biosorbent derived from alkaline lignin for lead removal from aqueous solution. Journal of Industrial and Engineering Chemistry, 23, 228–234.
Gonzalez Bermudez, Y., Rodriguez Rico, I. L., Gutierrez Bermudez, O., & Guibal, E. (2011). Nickel biosorption using Gracilaria caudata and Sargassum muticum. Chemical Engineering Journal, 166(1), 122–131.
Gupta, B. S., Curran, M., Hasan, S., & Ghosh, T. K. (2009). Adsorption characteristics of Cu and Ni on Irish peat moss. Journal of Environmental Management, 90(2), 954–960.
Jing, X., Liu, F., Yang, X., Ling, P., Li, L., Long, C., & Li, A. (2009). Adsorption performances and mechanisms of the newly synthesized N,N′-di (carboxymethyl) dithiocarbamate chelating resin toward divalent heavy metal ions from aqueous media. Journal of Hazardous Materials, 167(1–3), 589–596.
Kannamba, B., Reddy, K. L., & AppaRao, B. V. (2010). Removal of Cu(II) from aqueous solutions using chemically modified chitosan. Journal of Hazardous Materials, 175(1–3), 939–948.
Khabibi, J., Syafii, W., & Sari, R. K. (2016). Reducing hazardous heavy metal ions using mangium bark waste. Environmental Science and Pollution Research, 23(16), 16631–16640.
Khanbabaee, K., & van Ree, T. (2001). Tannins: classification and definition. Natural Product Reports, 18(6), 641–649.
Kim, Y., Ogata, T., & Nakano, Y. (2007). Kinetic analysis of palladium(II) adsorption process on condensed-tannin gel based on redox reaction models. Water Research, 41(14), 3043–3050.
Lakshtanov, L. Z., & Stipp, S. L. S. (2007). Experimental study of nickel(II) interaction with calcite: adsorption and coprecipitation. Geochimica et Cosmochimica Acta, 71(15), 3686–3697.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 40, 1361–1403.
Li, W., Tang, Y., Zeng, Y., Tong, Z., Liang, D., & Cui, W. (2012). Adsorption behavior of Cr(VI) ions on tannin-immobilized activated clay. Chemical Engineering Journal, 193, 88–95.
Li, Z., Xiao, D., Ge, Y., & Koehler, S. (2015a). Surface-functionalized porous lignin for fast and efficient lead removal from aqueous solution. ACS Applied Materials & Interfaces, 7(27), 15000–15009.
Li, Z., Kong, Y., & Ge, Y. (2015b). Synthesis of porous lignin xanthate resin for Pb2+ removal from aqueous solution. Chemical Engineering Journal, 270, 229–234.
Li, Z., Ge, Y., & Wan, L. (2015c). Fabrication of a green porous lignin-based sphere for the removal of lead ions from aqueous media. Journal of Hazardous Materials, 285, 77–83.
Maria Rahman, M., Akter, N., Karim, M. R., Ahmad, N., Rahman, M. M., Siddiquey, I. A., Bahadur, N. M., & Hasnat, M. A. (2014). Optimization, kinetic and thermodynamic studies for removal of Brilliant Red (X-3B) using Tannin gel. Journal of Environmental Chemical Engineering, 2(1), 76–83.
Matusik, J., & Wscislo, A. (2014). Enhanced heavy metal adsorption on functionalized nanotubular halloysite interlayer grafted with aminoalcohols. Applied Clay Science, 100(SI), 50–59.
Miretzky, P., & Fernandez Cirelli, A. (2010). Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: a review. Journal of Hazardous Materials, 180(1–3), 1–19.
Naczk, M., & Shahidi, F. (2007). Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis (vol 41, pg 1523, 2006). Journal of Pharmaceutical and Biomedical Analysis, 43(2), 798.
Nakano, Y., Takeshita, K., & Tsutsumi, T. (2001). Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel. Water Research, 35(2), 496–500.
Ngomsik, A. F., Bee, A., Siaugue, J. M., Cabuil, V., & Cote, G. (2006). Nickel adsorption by magnetic alginate microcapsules containing an extractant. Water Research, 40(9), 1848–1856.
Nuhoglu, Y., & Malkoc, E. (2009). Thermodynamic and kinetic studies for environmentaly friendly Ni(II) biosorption using waste pomace of olive oil factory. Bioresource Technology, 100(8), 2375–2380.
Pillai, S. S., Deepa, B., Abraham, E., Girija, N., Geetha, P., Jacob, L., & Koshy, M. (2013). Biosorption of Cd(II) from aqueous solution using xanthated nano banana cellulose: equilibrium and kinetic studies. Ecotoxicology and Environmental Safety, 98, 352–360.
Ping, L., Brosse, N., Chrusciel, L., Navarrete, P., & Pizzi, A. (2011). Extraction of condensed tannins from grape pomace for use as wood adhesives. Industrial Crops and Products, 33(1), 253–257.
Raval, N. P., Shah, P. U., & Shah, N. K. (2016). Adsorptive removal of nickel(II) ions from aqueous environment: a review. Journal of Environmental Management, 179, 1–20.
Reddy, D. H. K., Ramana, D. K. V., Seshaiah, K., & Reddy, A. V. R. (2011). Biosorption of Ni(II) from aqueous phase by Moringa oleifera bark, a low cost biosorbent. Desalination, 268(1–3), 150–157.
Samper, E., Rodriguez, M., De la Rubia, M. A., & Prats, D. (2009). Removal of metal ions at low concentration by micellar-enhanced ultrafiltration (MEUF) using sodium dodecyl sulfate (SDS) and linear alkylbenzene sulfonate (LAS). Separation and Purification Technology, 65(3), 337–342.
Sanchez-Martin, J., Beltran-Heredia, J., & Gragera-Carvajal, J. (2011a). Caesalpinia spinosa and Castanea sativa tannins: a new source of biopolymers with adsorbent capacity. Preliminary assessment on cationic dye removal. Industrial Crops and Products, 34(1), 1238–1240.
Sanchez-Martin, J., Beltran-Heredia, J., & Gibello-Perez, P. (2011b). Adsorbent biopolymers from tannin extracts for water treatment. Chemical Engineering Journal, 168(3), 1241–1247.
Sanchez-Martin, J., Beltran-Heredia, J., Seabra, I. J., Braga, M. E. M., & de Sousa, H. C. (2012). Adsorbent derived from Pinus pinaster tannin for cationic surfactant removal. Journal of Wood Chemistry and Technology, 32(1), 23–41.
Tan, P., Sun, J., Hu, Y., Fang, Z., Bi, Q., Chen, Y., & Cheng, J. (2015). Adsorption of Cu2+, Cd2+ and Ni2+ from aqueous single metal solutions on graphene oxide membranes. Journal of Hazardous Materials, 297, 251–260.
Thevannan, A., Mungroo, R., & Niu, C. H. (2010). Biosorption of nickel with barley straw. Bioresource Technology, 101(6), 1776–1780.
Vinod, V. T. P., Sashidhar, R. B., & Sreedhar, B. (2010). Biosorption of nickel and total chromium from aqueous solution by gum kondagogu (Cochlospermum gossypium): a carbohydrate biopolymer. Journal of Hazardous Materials, 178(1–3), 851–860.
Wang, X., Liu, Y., & Zheng, J. (2016). Removal of As(III) and As(V) from water by chitosan and chitosan derivatives: a review. Environmental Science and Pollution Research, 23(14), 13789–13801.
Yu, X. R., Liu, F., Wang, Z. Y., & Chen, Y. (1990). Auger parameters for sulfur-containing compounds using a mixed aluminum-silver excitation source. Journal of Electron Spectroscopy and Related Phenomena, 50(1–2), 159–166.
Yurtsever, M., & Sengil, I. A. (2009). Biosorption of Pb(II) ions by modified quebracho tannin resin. Journal of Hazardous Materials, 163(1), 58–64.
Zhan, X. M., Miyazaki, A., & Nakano, Y. (2001). Mechanisms of lead removal from aqueous solutions using a novel tannin gel adsorbent synthesized from natural condensed tannin. Journal of Chemical Engineering of Japan, 34(10), 1204–1210.
Zhao, P., Jiang, J., Zhang, F., Zhao, W., Liu, J., & Li, R. (2010). Adsorption separation of Ni(II) ions by dialdehyde o-phenylenediamine starch from aqueous solution. Carbohydrate Polymers, 81(4), 751–757.
Zhao, P., Ge, S., Chen, Z., & Li, X. (2013). Study on pore characteristics of flocs and sludge dewaterability based on fractal methods (pore characteristics of flocs and sludge dewatering). Applied Thermal Engineering, 58(1–2), 217–223.
Funding
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (Project No. 21677020)
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOCX 194 kb)
Rights and permissions
About this article
Cite this article
Zhao, C., Zheng, H., Sun, Y. et al. Fabrication of Tannin-Based Dithiocarbamate Biosorbent and Its Application for Ni(II) Ion Removal. Water Air Soil Pollut 228, 409 (2017). https://doi.org/10.1007/s11270-017-3593-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3593-0