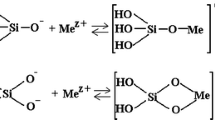
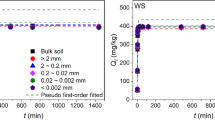
Abstract
The processes of pollutant sorption by soil components control their mobility, migration, and transformation in the soil-plant system and depend on numerous properties, among which the elemental composition and surface area are dominant. In a laboratory experiment, the sorption of As and Cd by Si-rich substances differing in surface area, solubility of Si, and mineralogical composition was studied. The adsorption data were fitted to the linear, logarithmic, exponential, Langmuir, and Freundlich equations. Both size of the particles and mineral solubility of Si affected the As and Cd sorption. In the systems with lower initial concentrations of As and Cd, size of the particles had more pronounced effect on the sorption capacity. In the systems with high initial concentrations of As and Cd, the concentration of monosilicic acid was more significant than the surface area.




Similar content being viewed by others
References
Balakhnina, T., & Borkowska, A. (2013). Effects of silicon on plant resistance to environmental stresses: review. International Agrophysics, 27, 225–232.
Bocharnikova, E. A., & Matichenkov, V. V. (2012). Influence of plant associations on the silicon cycle in the soil-plant ecosystem. Applied Ecology Environmental Research, 10(4), 547–560.
Bohn, H. L., Strawn, D. G., & O'Connor, G. A. (2015). Soil chemistry. New York, NY: Wiley.
Castaldi, P., Santona, L., Enzo, S., & Melis, P. (2007). Sorption processes and XRD analysis of a natural zeolite exchanged with Pb2+, Cd2+ and Zn2+ cations. Journal of Hazardous Materials, 156(1–3), 428–434.
Conway, G. R., & Pretty, J. N. (2013). Unwelcome harvest: agriculture and pollution. UK: Routledge.
Dinkelman, I.D. (2008). Removal of arsenic from waters with elevated concentration of silica using adsorptive processes. Dissertation Thesis, University of Nevada, Reno.
Duncan, D. B. (1957). Multiple range tests for correlated and heteroscedastic means. Biometrics, 13(2), 164–176.
Esfahani, A. R., Firouzi, A. F., Sayyad, G., & Kiasat, A. (2013). Isotherm study of cadmium adsorption onto stabilized-zerovalent iron nanoparticles. Journal of Agronomy and Plant Production, 4(12), 3444–3454.
Foo, K. Y., & Hameed, B. H. (2010). Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal, 156, 2–10.
Gu, X., Evans, L. J., & Barabash, S. J. (2010). Modeling the adsorption of Cd(II), Cu(II), Ni(II), Pb(II) and Zn(II) onto montmorillonite. Geochmica et Cosmochimica Acta, 74(20), 5718–5728.
He, B., Yun, Z., Shi, J., & Jiang, G. (2013). Research progress of heavy metal pollution in China: sources, analytical methods, status, and toxicity. Chinese Science Bulletin, 58(2), 134–140.
Iler, R. K. (1979). The chemistry of silica. New York, NY: Wiley.
Ji, X., Liu, S., Huang, J., Bocharnikova, E., & Matichenkov, V. (2016). Monosilicic acid potential in phytoremediation of the contaminated areas. Chemosphere, 157, 132–136.
Kumar, R., & Chawla, J. (2014). Removal of cadmium ion from water/wastewater by nano-metal oxides: a review. Water Quality, Exposure and Health, 5(4), 215–226.
Kumar, M., Ramanathan, A. L., Rahman, M. M., & Naidu, R. (2016). Concentrations of inorganic arsenic in groundwater, agricultural soils and subsurface sediments from the middle Gangetic plain of Bihar, India. Science of the Total Environment, 573, 1103–1114.
Ladonin, D. V. (2003). The effect of iron and clay minerals on the adsorption of copper, zinc, lead, and cadmium in the nodular horizon of podzolic soil. Eurasian Soil Science, 36(10), 1065–1073.
Li, Z., Ryan, J. A., Chen, J., & Al-Abed, S. R. (2001). Adsorption of cadmium on biosolids-amendded soils. Journal of Environmental Quallity, 30, 903–911.
Luxton, T. P., Eick, M. J., & Rimstidt, D. J. (2008). The role of silicate in the adsorption/desorption of arsenite on goethite. Chemical Geology, 252(3), 125–135.
Ma, J. F., & Takahashi, E. (2002). Soil, fertilizer, and plant silicon research in Japan. The Netherlands: Elsevier.
Matichenkov, V. V., & Bocharnikova, E. A. (1994). Silicon soil state and biogeochemical balance in forest and grass ecosystems. In Sustainable development: the view from the less industrialized countries (pp. 453–467). San Jose: UNED Press, Costa Rica.
Matichenkov, V. V., Bocharnikova, E. A., Pahnenko, E. P., Khomiakov, D. M., Matichenkov, I. V., Qiang, Z., & Xiao, W. (2016). Reduction of Cd, Cu, Ni, and Pb mobility by active Si in a laboratory study. Mine Water and Environment, 35(3), 302–309.
Mullin, J. B., & Riley, J. P. (1955). The colorimetric determination of silicate with reference to sea and natural waters. Analytica Chimica Acta, 12, 162–176.
Nguyen, T. C., Loganathan, P., Nguyen, T. V., Vigneswaran, S., Kandasamy, J., & Naidu, R. (2015). Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies. Chemical Engineering Journal, 270, 393–404.
Shaheen, S. M., & Tsadilas, C. D. (2010). Influence of fly ash and sewage sludge application on cadmium and lead sorption by an acidic Alfisol. Pedosphere, 20(4), 436–445.
Su, C., & Puls, R. W. (2001). Arsenate and arsenite removal by zerovalent iron: effects of phosphate, silicate, carbonate, borate, sulfate, chromate, molybdate, and nitrate, relative to chloride. Environmental Science & Technology, 35(22), 4562–4568.
Swedlund, P. J., & Webster, J. G. (1999). Adsorption and polymerization of silicic acid on ferrihydrite, and its effect on arsenic adsorption. Water Research, 33(16), 3413–3422.
Tang, W. W., Zeng, G. M., Gong, J. L., Liang, J., Xu, P., Zhang, C., & Huang, B. B. (2014). Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: a review. Science of the Total Environment, 468, 1014–1027.
Terasaki, O., Cho, H., Cho, M., Jeong, H., Asahina, S., Sakuda, Y., & Liu, Z. (2013). Novel structural characterizations of insulating and electron beam sensitive materials employing low voltage high resolution scanning electron microscopy. JEOL News, 48, 21–31.
Tirado, R., & Allsop, M. (2012). Phosphorus in agriculture: problems and solutions. Greenpeace Research Laboratories Technical Report (Review) 02–2012 (greenpeace.org).
Tripathi, D. K., Singh, V. P., Grangwar, S., Prasad, S. M., Maurya, J. N., & Chauhan, D. K. (2014). Role of silicon in enrichment of plant nutrients and protection from biotic and abiotic stresses. In P. Ahmad (Ed.), Improvement of crops in the era climatic changes (Vol. Volume 1, pp. 39–56). New York: Springer Science+Business Media.
Uddin, M. K. (2017). A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chemical Engineering Journal, 308, 438–462.
Vodyanitskii, Y. N., & Plekhanova, I. O. (2014). Biogeochemistry of heavy metals in contaminated excessively moistened soil (analytical review). Eurasian Soil Science, 47(3), 153–161.
Zhao, Q., Wang, Y., Cao, Y., Chen, A., Ren, M., Ge, Y., Yu, Z., Wan, S., Hu, A., Bo, Q., Ruan, L., Chen, H., Qin, S., Chen, W., Hu, C., Tao, F., Xu, D., Xu, J., Wen, L., & Li, L. (2014). Potential health risks of heavy metals in cultivated topsoil and grain, including correlations with human primary liver, lung and gastric cancer, in Anhui Province, Eastern China. Science of the Total Environment, 470-471, 340–347.
Acknowledgements
This study was supported by National Science Foundation of China (project 2013BAD03B02) and by Natural Science Foundation of Hunan Province in China (project 2016JJ6066).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, H., Ji, X., Wei, W. et al. As and Cd Sorption on Selected Si-Rich Substances. Water Air Soil Pollut 228, 288 (2017). https://doi.org/10.1007/s11270-017-3473-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3473-7