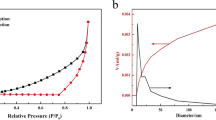
Abstract
Melamine, which has three free amino groups and three aromatic nitrogen atoms in its molecule, can be potentially used as an adsorbent for metal ions. Factors associated with adsorption efficiency of vanadium by melamine were systematically investigated, including initial pH value of solution, temperature, contact time, and dosage of melamine. The optimal operation conditions for adsorption performance of vanadium with melamine were obtained. The adsorption efficiency was over 99.97% at the initial pH value of 1.18, molar ratio of n (melamine)/n (vanadium) = 1.0 for 60 min. The kinetic data for the adsorption followed well the pseudo second-order kinetic model.







Similar content being viewed by others
References
Abbasi, S. M., & Shokuhfar, A. (2007). Improvement of mechanical properties of Cr-Ni-Mo-Cu-Ti stainless steel with addition of vanadium. Journal of Iron and Steel Research International, 14(6), 74–78.
Banwen, S., & Yuji, L. (1998). Inorganic chemistry series (vanadium, vol. 8). Beijing: Science press.
Chen, X., Lan, X., Zhang, Q., Ma, H., & Zhou, J. (2010). Leaching vanadium by high concentration sulfuric acid from stone coal. Transactions of the Nonferrous Metals Society of China, 20, 123–126.
Chen, D., Zhao, H., Hu, G., Qi, T., Yu, H., Zhang, G., Wang, L., & Wang, W. (2015). An extraction process to recover vanadium from low-grade vanadium-bearing titanomagnetite. Journal of Hazardous Materials, 294, 35–40.
Fan, Y., Wang, X., & Wang, M. (2013). Separation and recovery of chromium and vanadium from vanadium-containing chromate solution by ion exchange. Hydrometallurgy, 136, 31–35.
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34(5), 451–465.
Huang, M., Li, Z., Xie, Y., & Li, X. (2006a). Adsorptive performance of melamine for silver ions. Industrial Water Treatment, 26(1), 36–39.
Huang, M., Peng, Q., & Li, X. (2006b). Rapid and effective adsorption of lead ions on fine poly(phenylenediamine) microparticles. Chemistry-a European Journal, 12(14), 4341–4350.
Huang, M., Lu, H., & Li, X. (2012). Synthesis and strong heavy-metal ion sorption of copolymer microparticles from phenylenediamine and its sulfonate. Journal of Materials Chemistry, 22(34), 17685–17699.
Li, X., Huang, M., & Li, S. (2004). Facile synthesis of poly (1,8-diaminonaphthalene) microparticles with a very high silver-ion adsorbability by a chemical oxidative polymerization. Acta Materialia, 52(18), 5363–5374.
Li, X., Liu, R., & Huang, M. (2005). Facile synthesis and highly reactive silver ion adsorption of novel microparticles of sulfodiphenylamine and diaminonaphthalene copolymers. Chemistry of Materials, 17(22), 5411–5419.
Li, X., Ma, X., Sun, J., & Huang, M. (2009). Powerful reactive sorption of silver (I) and mercury (II) onto poly (o-phenylenediamine) microparticles. Langmuir, 25(3), 1675–1684.
Li, X., Feng, H., & Huang, M. (2010). Redox sorption and recovery of silver ions as silver Nanocrystals on poly(aniline-co-5-sulfo-2-anisidine) nanosorbents. Chemistry A European Journal, 16(33), 10113–10123.
Lin, L., Liu, K., Atsushi, S., Yen, W., Toyohisa, F., Osamu, S., & Akira, K. (2004). Recovery of tungsten and vanadium from tungsten alloy scrap. Hydrometallurgy, 72(1–2), 1–8.
Liu, Z., Nueraihemaiti, A., Chen, M., Du, J., Fan, X., & Tao, C. (2015). Hydrometallurgical leaching process intensified by an electric field for converter vanadium slag. Hydrometallurgy, 155, 56–60.
Lv, Q., Huang, M., & Li, X. (2007). Synthesis and heavy-metal-ion sorption of pure sulfophenylenediamine copolymer nanoparticles with intrinsic conductivity and stability. Chemistry-a European Journal, 13(21), 6009–6018.
Mazurek, K. (2013). Recovery of vanadium, potassium and iron from a spent vanadium catalyst by oxalic acid solution leaching, precipitation and ion exchange processes. Hydrometallurgy, 134-135, 26–31.
Moskalyk, R. R., & Alfantazi, A. M. (2003). Processing of vanadium: a review. Minerals Engineering, 16(9), 793–805.
Navarro, R., Guzman, J., Saucedo, I., Revilla, J., & Guibal, E. (2007). Vanadium recovery from oil fly ash by leaching, precipitation and solvent extraction processes. Waste Management, 27(3), 425–438.
Nguyen, T. H., & Lee, M. S. (2013). Separation of molybdenum and vanadium from acid solutions by ion exchange. Hydrometallurgy, 136, 65–70.
Nicholas, J. N., Silva, G. d., Kentish, S., & Stevens, G. W. (2014). Use of vanadium (V) oxide as a catalyst for CO2 hydration in potassium carbonate systems. Industrial & Engineering Chemistry Research, 53(8), 3029–3039.
Peng, H., Liu, Z., & Tao, C. (2015). Selective leaching of vanadium from chromium residue intensified by electric field. Journal of Environmental Chemical Engineering, 3(2), 1252–1257.
Peng, H., Liu, Z., & Tao, C. (2016a). Leaching kinetics of vanadium with electro-oxidation and H2O2 in alkaline medium. Energy & Fuels, 30(9), 7802–7807.
Peng, H., Liu, Z., & Tao, C. (2016b). Leaching of vanadium and chromium from residue. Journal of Research and Development, 4(1).
Peng, H., Liu, Z., & Tao, C. (2017). Adsorption kinetics and isotherm of vanadium with melamine. Water Science and Technology, 75(10), 2316–2321.
Sahu, K. K., Agrawal, A., & Mishra, D. (2013). Hazardous waste to materials: recovery of molybdenum and vanadium from acidic leach liquor of spent hydroprocessing catalyst using alamine 308. Journal of Environmental Management, 125(0), 68–73.
Saleta, M. E., Curiale, J., Troiani, H. E., Ribeiro, G. S., Sánchez, R. D., Malta, M., & Torresi, R. M. (2007). Magnetic characterization of vanadium oxide/polyaniline nanotubes. Applied Surface Science, 254(1), 371–374.
Wang, W. (2007). Continuous determination of vanadium and chromium in steel and alloy. Advanced Measurement and Laboratory Management, 4, 9–10.
Wei, Z., Liu, D., Hsu, C., & Liu, F. (2014). All-vanadium redox photoelectrochemical cell: an approach to store solar energy. Electrochemistry Communications, 45, 79–82.
Zeng, L., & Cheng, C. (2010). Recovery of molybdenum and vanadium from synthetic sulphuric acid leach solutions of spent hydrodesulphurisation catalysts using solvent extraction. Hydrometallurgy, 101(3–4), 141–147.
Zeng, L., Li, Q., Xiao, L., & Zhang, Q. (2010). A study of the vanadium species in an acid leach solution of stone coal using ion exchange resin. Hydrometallurgy, 105(1–2), 176–178.
Zhang, Y., Fan, B., & Peng, D. (2001). Research of precipitation poly ammonium vandate from extraction solution of acid leaching bone coal. Chinese Journal of Rare Metals, 02, 157–160.
Zhang, B., Hao, L., Tian, C., Yuan, S., Feng, C., Ni, J., & Borthwick, A. G. L. (2015). Microbial reduction and precipitation of vanadium (V) in groundwater by immobilized mixed anaerobic culture. Bioresource Technology, 192, 410–417.
Zhao, Z., Long, H., Li, X., Fan, Y., & Han, Z. (2012). Precipitation of vanadium from Bayer liquor with lime. Hydrometallurgy, 115-116, 52–56.
Zhao, Z., Guo, M., & Zhang, M. (2015). Extraction of molybdenum and vanadium from the spent diesel exhaust catalyst by ammonia leaching method. J Hazard Mater, 286.
Acknowledgements
This work was supported by Chongqing University Postgraduates’ Innovation Project (CYB15045) and the Natural Science Foundation of China (No. 51274261).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Peng, H., Liu, Z. & Tao, C. Adsorption Process of Vanadium (V) with Melamine. Water Air Soil Pollut 228, 272 (2017). https://doi.org/10.1007/s11270-017-3452-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3452-z