Abstract
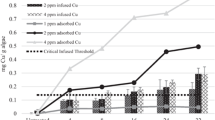
Efficacy of an in situ algaecide treatment can be predicted prior to an application by physically modeling exposures and responses with laboratory experiments. A sodium carbonate peroxyhydrate (SCP) algaecide was used in a drinking water reservoir (Hartwell Lake, Anderson, SC) to control a benthic algal assemblage putatively producing 2-methylisoboreol (MIB) and geosmin, compounds with adverse taste and odor attributes. These SCP applications provided an opportunity to test hypotheses regarding potential convergence of laboratory and in situ exposures and responses. Objectives of this study were to (1) measure responses of a benthic algal assemblage from Hartwell Lake to 7-day laboratory exposures of SCP [measured as hydrogen peroxide (H2O2) concentrations], (2) to measure the exposure of SCP (as H2O2) applied in a cove of Hartwell Lake and consequent responses of the algal assemblage, and (3) compare exposures and responses measured in the laboratory and in situ. Results demonstrated that in laboratory exposures, H2O2 released by SCP dissipated within 48 h. Significant responses of the algal assemblage in terms of phycocyanin concentrations and cell densities were measured 4 days after treatment (4-DAT) and 7-DAT following exposures of 453, 615, and 812 mg H2O2 m−2. The H2O2 exposure measured in situ was comparable to effective laboratory exposures in terms of initial exposure (619 ± 428 mg H2O2 m−2) and exposure duration (dissipation within 30 h), but the in situ exposure had a large deviation initially (i.e., ±428 mg H2O2 m−2) and was an order of magnitude less than the targeted initial exposure. Therefore, comparison of measured responses was critical to infer comparable exposures and confirm accuracy of the laboratory model. Significant in situ responses were measured 4-DAT and 7-DAT in terms of phycocyanin concentrations and cell densities, and were comparable to responses obtained from effective laboratory exposures (i.e., 453, 615, and 812 mg H2O2 m−2). Decreases in measured concentrations of MIB and geosmin at the intake of the drinking water treatment facility provided additional evidence that algae were sufficiently exposed to H2O2 from SCP. Results of this experiment provide evidence for the design and use of physical laboratory models to predict responses of algae in the field.






Similar content being viewed by others
References
American Public Health Association (APHA) (2012). Standard methods for the examination of water and wastewater (22nd ed.). Washington, DC: American Public Health Association.
Applied Biochemists (AB). (2007). Material safety data sheets. Milwaukee: Laporte Water Technologies and Biochem.
Barrington, D. J., & Ghadouani, A. (2008). Application of hydrogen peroxide for the removal of toxic cyanobacteria and other phytoplankton from wastewater. Environmental Science and Technology, 42, 8916–8921.
Barrington, D. J., Reichwaldt, E. S., & Ghadouani, A. (2013). The use of hydrogen peroxide to remove cyanobacteria and microcystins from waste stabilization ponds and hypereutrophic systems. Ecological Engineering, 50, 86–94.
Barroin, G., & Feuillade, M. (1986). Hydrogen peroxide as a potential algicide for Oscillatoria rubescens DC. Water Research, 20(5), 619–623.
Bishop, W. M., & Rodgers, J. H., Jr. (2011). Responses of Lyngbya magnifica Gardner to an algaecide exposure in the laboratory and field. Ecotoxicology and Environmental Safety, 74, 1832–1838.
Burson, A., Matthijs, H. C. P., De Bruijne, W., Talens, R., Hoogenboom, R., Gerssen, A., Visser, P. M., Stomp, M., Steur, K., Van Scheppingen, Y., & Huisman, J. (2014). Termination of a toxic Alexandrium bloom with hydrogen peroxide. Harmful Algae, 31, 125–135.
Calomeni, A. J., & Rodgers, J. H., Jr. (2015). Evaluation of the utility of six measures for algal (Microcystis aeruginosa, Planktothrix agardhii and Pseudokirchneriella subcapitata) viability. Ecotoxicology and Environmental Safety, 111, 192–198.
Drabkova, M., Admiraal, W., & Marsalek, B. (2007a). Combined exposure to hydrogen peroxide and light—selective effects on cyanobacteria, green algae, and diatoms. Environmental Toxicology and Chemistry, 41, 309–314.
Drabkova, M., Matthijs, H. C. P., Admiraal, W., & Marsalek, B. (2007b). Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica, 45, 363–369.
Finnegan, M., Linley, E., Denyer, S. P., McDonnell, G., Simons, C., & Maillard, J. Y. (2010). Mode of action of hydrogen peroxide and other oxidizing agents: differences between liquid and gas forms. The Journal of Antimicrobial Chemotherapy, 65, 2108–2115.
Fitzgerald, G. P., & Jackson, D. F. (1979). Comparative algicide evaluations using laboratory and field algae. Journal of Aquatic Plant Management, 17, 66–71.
Geer, T. D., Kinley, C. M., Iwinski, K. J., Calomeni, A. J., & Rodgers, J. H., Jr. (2016). Comparative toxicity of sodium carbonate peroxyhydrate to freshwater organisms. Ecotoxicology and Environmental Safety, 132, 202–211.
Gettys, L. A., Haller, W. T., & Bellaud, M. (2014). Biology and control of aquatic plants. Marietta: Aquatic Ecosystem Restoration Foundation.
Human & Environmental Risk Assessment (HERA) (2002). Sodium percarbonate. Accessed 15 March 2016.
Kinley, C. M., Rodgers, J. H., Jr., Iwinski, K. J., McQueen, A. D., & Calomeni, A. J. (2015). Analysis of algaecide exposures: an evaluation of the I3 − method to measure sodium carbonate peroxyhydrate algaecides. Water, Air, and Soil Pollution, 226(6), 1–9.
Klassen, N. V., Marchington, D., & McGowan, H. C. E. (1994). Hydrogen peroxide determination by the I3 − method and by KMnO4 titration. Analytical Chemistry, 66, 2921–2925.
Lawrenz, E., Fedewa, E. J., & Richardson, T. L. (2011). Extraction protocols for the quantification of phycobilins in aqueous phytoplankton extracts. Journal of Applied Phycology, 24, 865–871.
Mallick, N., & Mohn, F. H. (2000). Reactive oxygen species: response of algal cells. Journal of Plant Physiology, 157, 183–193.
Matthijs, H. C. P., Visser, P. M., Reeze, B., Meeuse, J., Slot, P. C., Wijn, G., Talens, R., & Huisman, J. (2012). Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Research, 46, 1460–1472.
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7(9), 405–410.
Organization for Economic Cooperation and Development (OECD) (2006). Screening Information Data Set (SIDS): Sodium percarbonate. Paris, France.
Palmer, C. M. (1960). Algae and other interference organisms in the waters of the South Central United States. Journal of the American Water Works Association, 52(7), 897–914.
Porter, S. D., Cuffney, T. F., Gurtz, M. E., & Meador, M. R. (1993). Methods for collecting algal samples as part of the national water-quality assessment program. U.S. Geological Survey Open-File Report 93-409. Raleigh: US Geological Survey.
Rand, G. M. (Ed.). (1995). Fundamentals of aquatic toxicology: effects, environmental fate and risk assessment. Washington, DC: Taylor and Francis.
Ridal, J. J., Watson, S. B., & Hickey, M. B. C. (2007). A comparison of biofilms from macrophytes and rocks for taste and odour producers in the St. Lawrence river. Water Science and Technology, 55(5), 15–21.
Rodgers, J. H., Jr., Johnson, B. M., & Bishop, W. M. (2010). Comparison of three algaecides for controlling the density of Prymnesium parvum. Journal of the American Water Resources Association, 46, 153–160.
Stevenson, R. J., & Bahls, L. L. (1999). Periphyton protocols. In M. T. Barbour, J. Gerristen, B. D. Snyder, & J. B. Stribling (Eds.), Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish, second edition (chapter 6). EPA 841-B-99-002. Washington, D.C: U.S. Environmental Protection Agency, Office of Water.
United States Army Corps of Engineers (USACE) (1992). Authorized and operating purposes of corps of engineers reservoirs. OMB No. 0704-0188.. Davis: Institute for water resources hydrologic engineering center.
United States Environmental Protection Agency (USEPA) (1996a). Ecological effects test guidelines. OPPTS 850.1075 Fish acute toxicity test, freshwater and marine. Prevention, pesticides, and toxic substances (7101). EPA 712-C-96-114.
United States Environmental Protection Agency (USEPA) (1996b). Ecological Effects Test Guidelines. OPPTS 850.1010 Aquatic invertebrate acute toxicity test, freshwater daphnids. Prevention, pesticides, and toxic substances (7101). EPA 712-C-96-114.
United States Environmental Protection Agency (USEPA) (2004). Registration Eligibility Decision (RED). PAKTM 27. Human and ecological risk assessment for section 3 registration of the end-use product PAKTM 27 for application to lakes, ponds, and drinking water reservoirs. EPA registration no. 68660-9-67690. Office of pesticide programs, biopesticides and pollution prevention division, Washington, DC.
Utkilen, H. C., & Frøshaug, M. (1992). Geosmin production and excretion in a planktonic and benthic Oscillatoria. Water Science and Technology, 25(2), 199–206.
Vilalta, E., Guasch, H., Muñoz, I., Romani, A., Valero, F., Rodriguez, J. J., Alcaraz, R., & Sabater, S. (2004). Nuisance odours produced by benthic cyanobacteria in a Mediterranean river. Water Science and Technology, 49(9), 25–31.
Watson, S. B., & Ridal, J. (2004). Periphyton: a primary source of widespread and severe taste and odou. Water Science and Technology, 49(9), 33–39.
Acknowledgements
The authors thank LONZA Group Ltd. for funding this research. We also thank Dr. Ryan Wersal for review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geer, T.D., Calomeni, A.J., Kinley, C.M. et al. Predicting In Situ Responses of Taste- and Odor-Producing Algae in a Southeastern US Reservoir to a Sodium Carbonate Peroxyhydrate Algaecide Using a Laboratory Exposure-Response Model. Water Air Soil Pollut 228, 53 (2017). https://doi.org/10.1007/s11270-016-3229-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3229-9