Abstract
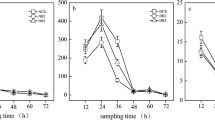
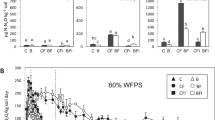
The aim of this study was to investigate the effect of biochar addition on the denitrification process and N2O emission in Cd-contaminated soil. Four different biochars, i.e., dairy manure and rice straw pyrolyzed at 350 and 550 °C, respectively, were added into a Cd-contaminated soil and incubation experiments were conducted for 8 weeks. Results showed that Cd had an inhibitory effect on denitrifying reductase enzymes and reduced the abundance of functional genes. On the contrary, amendment with the biochars increased denitrifying enzyme activity and gene abundance, and thus, enhanced the denitrification process. Labile carbon (C) in the biochar-amended soil, which was calculated based on the two-pool exponential model, was the key factor to facilitate this process. As a less important factor, elevated soil pH by biochar addition also increased denitrifying activity as well as the nosZ abundance. Decrease of Cd bioavailability by the biochar addition was beneficial to the denitrification process. Addition of the biochars with higher amount of NO3 −-N, especially the rice straw-derived biochars, increased cumulative N2O emission by more than ten times relative to the Cd-contaminated soil. With the great amount of labile C and NO3 −-N, the treatment of biochars prepared at 350 °C released the larger amount of CO2 and N2O than other treatments. The biochar addition could totally release the heavy metal stress and restore the Cd-contaminated soil in terms of bacterial community.






Similar content being viewed by others
References
Adriano, D. C. (2001). Trace elements in the terrestrial environments: biogeochemistry, bioavailability, and risks of heavy metals. New York: Springer.
Ameloot, N., De Neve, S., Jegajeevagan, K., Yildiz, G., Buchan, D., Funkuin, Y. N., Prins, W., Bouckaert, L., & Sleutel, S. (2013). Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biology & Biochemistry, 57, 401–410.
Ameloot, N., Maenhout, P., De Neve, S., & Sleutel, S. (2016). Biochar-induced N2O emission reductions after field incorporation in a loam soil. Geoderma, 267, 10–16.
Baggs, E. M., Rees, R. M., Smith, K. A., & Vinten, A. J. A. (2006). Nitrous oxide emission from soils after incorporating crop residues. Soil Use and Management, 16(2), 82–87.
Baggs, E. M., Smales, C. L., & Bateman, E. J. (2010). Changing pH shifts the microbial source as well as the magnitude of N2O emission from soil. Biology and Fertility of Soils, 46(8), 793–805.
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., Fierer, N., Pena, A. G., Goodrich, J. K., & Gordon, J. I. (2010). QIIME allows analysis of high-throughput community sequencing data. Nature Methods, 7(5), 335–336.
Cayuela, M. L., Jeffery, S., & van Zwieten, L. (2015). The molar H:Corg ratio of biochar is a key factor in mitigating N2O emissions from soil. Agriculture, Ecosystems & Environment, 202, 135–138.
Cayuela, M.L., Oenema, O., Kuikman, P.J., Bakker, R.R., & Van Groenigen, J. W. (2010). Bioenergy by-products as soil amendments? Implications for carbon sequestration and greenhouse gas emissions. GCB Bioenergy 2: 201–213.
Cayuela, M. L., van Zwieten, L., Singh, B. P., Jeffery, S., Roig, A., & Sánchez-Monedero, M. A. (2014). Biochar’s role in mitigating soil nitrous oxide emissions: a review and meta-analysis. Agriculture, Ecosystems & Environment, 191, 5–16.
Cui, L., Yan, J., & Yang, Y. (2013). Influence of biochar on microbial activities of heavy metals contaminated paddy fields. BioResources, 8, 5536–5548.
Dong, D., Feng, Q., McGrouther, K., Yang, M., Wang, H., & Wu, W. (2014). Effects of biochar amendment on rice growth and nitrogen retention in a waterlogged paddy field. Journal of Soils and Sediments, 15, 153–162.
Ducey, T. F., Ippolito, J. A., Cantrell, K. B., Novak, J. M., & Lentz, R. D. (2013). Addition of activated switchgrass biochar to an aridic subsoil increases microbial nitrogen cycling gene abundances. Applied Soil Ecology, 65, 65–72.
Gao, S., Zhao, M., Chen, Y., Yu, M., & Ruan, W. (2015). Tolerance response to in situ ammonia stress in a pilot-scale anaerobic digestion reactor for alleviating ammonia inhibition. Bioresource Technology, 198, 372–379.
Garau, G., Castaldi, P., Santona, L., Deiana, P., & Melis, P. (2007). Influence of red mud, zeolite and lime on heavy metal immobilization, culturable heterotrophic microbial populations and enzyme activities in a contaminated soil. Geoderma, 142(1-2), 47–57.
Harter, J., Krause, H. M., Schuettler, S., Ruser, R., Fromme, M., Scholten, T., Kappler, A., & Behrens, S. (2014). Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME Journal, 8(3), 660–674.
Helder, W., & De Vries, R. T. P. (1979). An automatic phenol-hypochlorite method for the determination of ammonia in sea-and brackish waters. Netherlands Journal of Sea Research, 13(1), 154–160.
Huang, C., Li, Z., Chen, F., Liu, Q., Zhao, Y., Zhou, J., & Wang, A. (2015). Microbial community structure and function in response to the shift of sulfide/nitrate loading ratio during the denitrifying sulfide removal process. Bioresource Technology, 197, 227–234.
Jones, D. L., Murphy, D. V., Khalid, M., Ahmad, W., Edwards-Jones, G., & DeLuca, T. H. (2011). Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biology & Biochemistry, 43(8), 1723–1731.
Khodadad, C. L. M., Zimmerman, A. R., Green, S. J., Uthandi, S., & Foster, J. S. (2011). Taxa-specific changes in soil microbial community composition induced by pyrogenic carbon amendments. Soil Biology & Biochemistry, 43(2), 385–392.
Lam, P., & Kuypers, M. M. (2011). Microbial nitrogen cycling processes in oxygen minimum zones. Annual Review of Marine Science, 3, 317–345.
Larson, C. (2014). China gets serious about its pollutant-laden soil. Science, 343(6178), 1415–1416.
Lehmann, J., Rillig, M. C., Thies, J., Masiello, C. A., Hockaday, W. C., & Crowley, D. (2011). Biochar effects on soil biota—a review. Soil Biology & Biochemistry, 43(9), 1812–1836.
Liu, B., Morkved, P. T., Frostegard, A., & Bakken, L. R. (2010). Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiology Ecology, 72(3), 407–417.
Lozupone, C., Hamady, M., & Knight, R. (2005). UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics, 241(110), 184–186.
Lu, H., Li, Z., Fu, S., Mendez, A., Gasco, G., & Paz-Ferreiro, J. (2015). Combining phytoextraction and biochar addition improves soil biochemical properties in a soil contaminated with Cd. Chemosphere, 119, 209–216.
Magalhães, C., Costa, J., Teixeira, C., & Bordalo, A. A. (2007). Impact of trace metals on denitrification in estuarine sediments of the Douro River estuary, Portugal. Marine Chemistry, 107(3), 332–341.
Magalhães, C. M., Machado, A., Matos, P., & Bordalo, A. A. (2011). Impact of copper on the diversity, abundance and transcription of nitrite and nitrous oxide reductase genes in an urban European estuary. FEMS Microbiology Ecology, 77(2), 274–284.
Martinez-Alonso, M., Escolano, J., Montesinos, E., & Gaju, N. (2010). Diversity of the bacterial community in the surface soil of a pear orchard based on 16S rRNA gene analysis. International Microbiology, 13(3), 123–134.
McKenney, D., & Vriesacker, J. (1985). Effect of cadmium contamination on denitrification processes in Brookston clay and Fox sandy loam. Environmental Pollution Series A, Ecological and Biological, 38(3), 221–233.
McMillan, A. M. S., Pal, P., Phillips, R. L., Palmada, T., Berben, P. H., Jha, N., Saggar, S., & Luo, J. (2016). Can pH amendments in grazed pastures help reduce N2O emissions from denitrification?—The effects of liming and urine addition on the completion of denitrification in fluvial and volcanic soils. Soil Biology & Biochemistry, 93, 90–104.
Miller, M. N., Zebarth, B. J., Dandie, C. E., Burton, D. L., Goyer, C., & Trevors, J. T. (2008). Crop residue influence on denitrification, N2O emissions and denitrifier community abundance in soil. Soil Biology & Biochemistry, 40(10), 2553–2562.
Mora, A. P. D., Ortega-Calvo, J. J., Cabrera, F., & Madejón, E. (2005). Changes in enzyme activities and microbial biomass after “in situ” remediation of a heavy metal-contaminated soil. Applied Soil Ecology, 28(2), 125–137.
Nelissen, V., Rütting, T., Huygens, D., Staelens, J., Ruysschaert, G., & Boeckx, P. (2012). Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biology & Biochemistry, 55, 20–27.
Ridnour, L. A., Sim, J. E., Hayward, M. A., Wink, D. A., Martin, S. M., Buettner, G. R., & Spitz, D. R. (2000). A spectrophotometric method for the direct detection and quantitation of nitric oxide, nitrite, and nitrate in cell culture media. Analytical Biochemistry, 281(2), 223–229.
Sørensen, J. (1978). Denitrification rates in a marine sediment as measured by the acetylene inhibition technique. Applied and Environmental Microbiology, 36(1), 139–143.
Scheer, C., Grace, P. R., Rowlings, D. W., Kimber, S., & Van Zwieten, L. (2011). Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia. Plant and Soil, 345(1–2), 47–58.
Schlegel, H. G. (1985). Allgemeine Mikrobiologie. Stuttgart: Georg Thieme Verlag.
Singh, D. K., & Kumar, S. (2008). Nitrate reductase, arginine deaminase, urease and dehydrogenase activities in natural soil (ridges with forest) and in cotton soil after acetamiprid treatments. Chemosphere, 71(3), 412–418.
Spokas, K. A., & Reicosky, D. C. (2009). Impacts of sixteen different biochars on soil greenhouse gas production. Annals of Environmental Science, 3(1), 4.
Suzuki, S., Kataoka, K., & Yamaguchi, K. (2000). Metal coordination and mechanism of multicopper nitrite reductase. Accounts of Chemical Research, 33(10), 728–735.
Troy, S. M., Lawlor, P. G., O’ Flynn, C. J., & Healy, M. G. (2013). Impact of biochar addition to soil on greenhouse gas emissions following pig manure application. Soil Biology & Biochemistry, 60, 173–181.
Uchimiya, M., Wartelle, L. H., Klasson, K. T., Fortier, C. A., & Lima, I. M. (2011). Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. Journal of Agricultural and Food Chemistry, 59(6), 2501–2510.
Urich, T., Lanzén, A., Qi, J., Huson, D. H., Schleper, C., & Schuster, S. C. (2008). Simultaneous assessment of soil microbial community structure and function through analysis of the meta-transcriptome. PLoS ONE, 3(6), e2527.
Van Zwieten, L., Singh, B. P., Kimber, S. W. L., Murphy, D. V., Macdonald, L. M., Rust, J., & Morris, S. (2014). An incubation study investigating the mechanisms that impact N2O flux from soil following biochar application. Agriculture, Ecosystems & Environment, 191, 53–62.
Vasquezmurrieta, M., Cruzmondragon, C., Trujillotapia, N., Herreraarreola, G., Govaerts, B., Vancleemput, O., & Dendooven, L. (2006). Nitrous oxide production of heavy metal contaminated soil. Soil Biology & Biochemistry, 38(5), 931–940.
Vig, K. (2003). Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review. Advances in Environmental Research, 8(1), 121–135.
Xu, H., Wang, X., Li, H., Yao, H., Su, J., & Zhu, Y. (2014). Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environmental Science & Technology, 48(16), 9391–9399.
Yang, F., Cao, X., Gao, B., Zhao, L., & Li, F. (2015). Short-term effects of rice straw biochar on sorption, emission, and transformation of soil NH(4)(+)-N. Environmental Science and Pollution Research International, 22, 9184–9192.
Zhang, H., Voroney, R., & Price, G. (2015). Effects of temperature and processing conditions on biochar chemical properties and their influence on soil C and N transformations. Soil Biology & Biochemistry, 83, 19–28.
Zumft, W. G. (1997). Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews, 61, 533–616.
Acknowledgements
This study was partly supported by grant from the Chinese National Natural Science Foundation (No. 41471181).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, G., Zhang, Z., Zhang, Z. et al. Influence of Biochar Addition on the Denitrification Process and N2O Emission in Cd-Contaminated Soil. Water Air Soil Pollut 228, 47 (2017). https://doi.org/10.1007/s11270-016-3228-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3228-x